-
IPCC第5次报告指出: 到本世纪末, 全球地表平均温度可能会升高1.5~2℃[1]。气温增加会导致土壤温度增加和土壤湿度的下降[2-3]。温度和水分作为调节生态系统过程中最重要的两个环境因子, 二者变化会深刻地影响着土壤生物学过程(如: 土壤有机质分解)[4]。土壤有机质分解作为土壤生物化学过程中重要的一步, 它是连接陆地生态系统碳氮循环的重要环节[5]。土壤酶通过酶促反应打破土壤有机质的复杂结构, 将复杂化合物转化为简单化合物以供微生物分解利用。土壤环境因子引起土壤酶库(糖苷酶、磷酸酶、蛋白酶等)变化是影响土壤有机碳分解的主要原因[6-7]。土壤酶作为土壤细菌、真菌和古菌的代谢产物[8], 环境因子通过影响微生物群落结构和生理作用[9-10], 进而改变土壤酶活性。胞外酶活性作为土壤微生物功能的指示因子, 已成为检验土壤微生物对气候变化响应的重要工具[11]。目前关于模拟气候变暖对土壤酶活性影响的研究中关于农田和草原的报道较多, 关于森林的报道相对较少。Wang等人[12]通过研究高山草甸发现增温处理对土壤纤维素酶、过氧化氢酶和磷酸酶活性无显著影响, 但显著提高了脲酶活性。Zhou等人[13]通过对北方草原研究发现增温处理显著增加了磷酸酶活性。Steinweg[14]通过对弃耕农田增温试验发现增温并未改变土壤酶(BG、CB、NAG)活性, 且土壤酶活性并未随着季节性温度变化而发生改变。McDaniel[15]通过对采伐迹地进行增温试验发现: 增温处理显著降低了BG和NAG酶活性, 且与土壤碳库和氮库相比, 非生物因子是调控土壤酶活性的主要原因。不同的研究结果表明调控土壤酶活性的生态因子是复杂的, 这也为利用土壤酶准确地检验土壤微生物对气候变化响应增加了不确定性。因此研究变化环境下的土壤酶活性对于探索土壤碳库对气候变化的响应及气候、生态系统和大气CO2浓度间的反馈作用具有重要的意义[16]。
河南省宝天曼国家级自然保护区位于北亚热带向南暖温带过渡区域, 属气候变化敏感区。Luan等人在此研究表明: 温度是影响土壤呼吸的重要环境因子, 土壤异氧呼吸的温度敏感性高于自养呼吸[17], 说明该区域微生物对气候变暖的响应更敏感, 预测未来气候变暖背景下该区微生物分解作用导致土壤碳库更容易分解。通过海拔土柱置换试验模拟气候变暖发现气候变暖显著增加了土壤CO2通量并改变土壤有机质层的分解过程[18]。本研究以该区域落叶阔叶林土壤为研究对象, 采用红外辐射增温模拟气候变暖进行进一步研究。旨在探明: (1)模拟气候变化背景下, 土壤酶活性季节变化规律; (2)土壤酶活性对环境因子变异的响应, 以深入理解气候变化背景下微生物因素在调控土壤碳循环过程中的作用。
HTML
-
研究地位于河南省南阳市内乡县宝天曼国家级自然保护区(111°47′~112°04′ E, 33°20′~33°36′ N), 海拔600~1 800 m, 是北亚热带向南暖温带过渡区域, 年均气温15.1℃, 年均降水量为885.6 mm, 降水多集中分布于6—8月。土壤垂直分布明显, 海拔1 300 m以上为山地棕壤, 海拔800~1 300 m为山地黄棕壤, 海拔600~800 m为山地褐土, 土壤厚度20~60 cm[19]。试验区植被以锐齿栎(Quercus aliena var. acuteserrata Maxim.)为优势树种, 占林分的65%, 林龄60年, 林分密度1 276.7株·hm-2; 其他伴生树种主要有四照花(Dendrobenthamia japonica (A. P. DC.) Fang var. chinensis (Osborn) Fang), 青皮槭(Acer cappadocicum Gled.)等[20]。
-
采用随机区组试验设计, 于2011年3月在研究区域典型林分中布设3个区组, 具体试验设计见文献[20]。简言之: 在增温样地内, 将长165 cm, 宽15 cm的红外线辐射加热灯(MSR-2420, Kalglo Electronics Inc., Bethlehem, PA, USA)平行样地短边垂直悬挂于样地正中央上方, 悬挂高度2.25 m, 额定功率为2 000瓦, 全年持续供电; 在对照样地内, 以相同的方式安装与红外线辐射加热灯形状和大小相同的假灯, 以消除红外线辐射加热灯遮阴对试验造成的误差。
-
2013年7月和12月进行野外土壤样品采集, 采样前清除土壤表面新鲜和半分解的凋落物残体, 在增温和对照处理每个子样地内随机选择3个点, 利用直径5.8 cm土钻采集0~10 cm土层的土壤样品混合, 并对采样点进行标识。采集的鲜土用冰盒保温, 医用泡沫箱封存好迅速带回实验室过2 mm土筛去除碎石和植物残体等, 一部分鲜土置于4℃冰箱中保存以待后期测定土壤微生物量碳氮、酶活性、硝态氮和铵态氮含量; 另一部分鲜土室内风干后用于pH测定, 过100目筛后用于总有机碳和全氮测定。采样时同步测定土壤温度和湿度: 5 cm土壤湿度(SWC)由MPK便携式土壤水分仪(MPKit, Nantong, China)测得, 每个子样地随机选取3个点测量取平均值; 同时利用便携式土壤温度计(Spectrun, USA)测定5 cm土壤温度, 每个子样地随机选取3个点测量取平均值。
-
土壤酶测定: 把相当于1 g干土的鲜土或1.25 g鲜土加入125 mL的醋酸钠缓冲液(50 mmol · L-1, pH=5.0)中, 在搅拌机中处理1 min制成均质土壤悬液。水解酶测定采用荧光微平板法[21]: 样品控制(800 μL土壤悬液+200 μL酶底物), 对照控制(800 μL土壤悬液+200 μL缓冲液), 淬火控制(800 μL土壤悬液+200 μL标准液), 底物控制(控制1∶200 μL缓冲液+50 μL酶底物, 控制2∶200 μL缓冲液+50 μL标准底物), 以上操作除底物控制加入96孔板(黑板)外, 其余均加入96深孔板中; 在25℃培养箱中暗培养3 h后加入10 μL NaOH(0.5 mol · L-1)停止反应, 将96深孔板以5 000 r·min-1离心3 min后, 用八通道微量取样器取250 μL加到96孔板中(黑板), 标仪(PerkinElmer Enspire, USA)进行荧光扫描(波长: 365 nm, 450 nm)。氧化酶测定采用比色法: 以25 mmol·L-1 L-DOPA作为底物, 酚氧化酶测定中样品控制(800 μL土壤悬液+200 μLDOPA), 对照控制(800 μL土壤悬液+200μL缓冲液), 底物控制(200 μL缓冲液+50 μLDOPA); 过氧化物酶测定中样品控制(800 μL土壤悬液+200 μL DOPA+40 μLH2O2), 对照控制(800 μL土壤悬液+200 μL缓冲液+40 μL H2O2), 底物控制(200 μL缓冲液+50 μL DOPA+10 μL H2O2)(H2O2浓度3%)。以上操作除底物控制加入96孔板(黑板)外, 其余均加入96深孔板中。室温震荡暗培养1 h后, 将96深孔板以5 000 r·min-1离心3 min后, 用八通道微量取样器取250 μL加到96孔板中(黑板), 酶标仪(PerkinElmer Enspire, USA)进行吸光扫描(波长: 465 nm)。(底物信息见表 1, 酶活性计算方法见文献[22])
中文名
Chinese Name缩写
abbreviation功能
Function底物
Substrate浓度
Concentration/ (μmol · L-1)α-葡糖苷酶 AG 碳循环: 从可溶性糖中释放葡萄糖 4-MUB-α-D-glucoside 20(酶底物) β-葡糖苷酶 BG 碳循环: 从纤维素中释放葡萄糖 4-MUB-β-D-glucoside 20(酶底物) 维二糖水解酶 CB 碳循环: 从纤维素中释放二糖 4-MUB-β-D-cellobioside 20(酶底物) N-乙酰-葡糖苷酶 NAG 氮循环: 降解几丁质 4-MUB-N-acetyl-β-D-glucosaminide 20(酶底物) 蛋白酶 LAP 氮循环: 把蛋白质降解成氨基酸 L-Leucine-7-amido-4-methylcoumarin 20(酶底物) 4-甲基伞形酮 MUB 4-methylumbelliferone 10(标准底物) 7-氨基-4-甲基伞形酮 AMC 7-amino-4-methylcoumarin 10(标准底物) 酚氧化物酶 POX 碳循环: 降解难分解物质 L-DOPA 25 000 过氧化物酶 PER 碳循环: 降解难分解物质 L-DOPA 25 000 Table 1. The substrate basic information
土壤理化性质测定: 土壤有机碳含量采用重铬酸钾外加热方法测定[23], 土壤全氮含量采用凯氏定氮方法测定[24]; 土壤铵态氮、硝态氮含量通过2 mol KCL溶液提取过滤后采用流动分析仪测定[25]; 土壤pH采用玻璃电极法测定[26]; 微生物量碳氮采用氯仿熏蒸浸提法[27]。
-
文章中所有酶活性均采用土壤专一酶活性, 专一酶活性=土壤酶活性/微生物量碳[28]。采用水热配比(S/T)作为衡量土壤环境因子的指标(S/T=平均土壤湿度/平均土壤温度)。采用t检验比较增温处理和对照处理土壤理化性质、生长季和非生长季内增温处理和对照处理土壤活性碳库、氮库和专一酶活性以及相同处理下生长季和非生长季专一酶活性的差异。所有的数据都使用SPSS 17.0进行统计分析, 图表采用Sigmaplot 12.5绘制, 用Canoco 4.5分析专一土壤酶活性与环境因子间的关系。
2.1. 样地设置
2.2. 土壤样品采集及土壤温湿度测定
2.3. 土壤酶活性及理化性质测定
2.4. 数据统计分析
-
表 2可知: 与对照样地相比, 增温处理土壤有机碳含量显著低于对照处理(P < 0.05), 土壤有机碳含量降低12.15%;增温处理土壤总氮和pH值虽低于对照处理, 但未达到显著水平; 增温与对照处理对土壤碳氮比的影响也未达到显著水平。
处理Treatment 有机碳SOC/(g · kg1) 总氮TN/(g · kg-1) 碳氮比SOC/TN pH值pH value 对照Ambient 52.79 ±1.00a 3.72 ±0.32a 14.36 ± 1.02a 4.46 ±0.11a 增温Warming 46.38 ±1.89b 3.17 ±0.26a 14.93 ± 1.86a 4.34 ± 0.07a (不同字母表示在0.05水平上显著差异)
(The different letters refer to significant effects at p < 0.05)Table 2. Soil physical and chemical properties
-
表 3和4表明: 生长季和非生长季中, 增温处理显著改变土壤环境, 增温处理显著增加土壤温度和降低土壤湿度导致土壤水热配比显著低于对照样地, 且增温处理导致非生长季水热配比下降幅度大于生长季的下降幅度; 土壤溶解性有机碳含量在生长季和非生长季对增温处理的响应均未达到显著水平; 生长季增温处理土壤微生物量碳和微生物量氮显著高于对照样地, 分别增加了40.30%和61.29%, 微生物量碳氮比显著低于对照样地12.64%;非生长季增温处理对土壤微生物量碳、微生物量氮和微生物量碳氮比的影响并不显著; 土壤硝态氮和铵态氮含量在生长季和非生长季对增温处理的响应均未达到显著水平。
季节Season 处理Treatment 水热比S/T 溶解性有机碳DOC/(mg · g-1) 微生物量碳MBC/(mg · kg-1) 微生物量氮MBN/(mg · kg-1) 微生物碳氮比MBC/MBN 硝态氮N03-N/(mg · g-1) 铵态氮NH4-N/(mg · g-1) 生长季Growing season 对照Ambient 1.52±0.11a 0.32 ± 0.08a 588.03 ±58.04b 77.98 ±7.26b 7.53 ± 0.24a 3.08 ± 0.98a 53.99 ±3.95a 增温Warming 1.11 ±0.04b 0.25 ±0.02a 825.03 ±17.42a 125.78 ±5.79a 6.58 ± 0.06b 3.29 ± 1.08a 44.76 ±9. 80a 非生长季Ungrowing season 对照Ambient 7.06±0.29a 0.17±0.03a 670.23 ±114.19a 117.85 ±17.2a 5.68 ± 0.35a 4.04 ± 1.59a 11.96 ± 1.39a 增温Warming 2.65 ± 0.50b 0.17±0.01a 607.59 ± 26.67a 103.70 ±2.94a 5. 86 ± 0.12a 5.54 ±0.70a 14.82 ±3.16a (不同字母表示在0.05水平上显著差异)
(The different letters refer to significant effects at p < 0.05)Table 3. Results of t-test on soil labile carbon and nitrogen pools and environment
季节
Season处理
Treatment土壤温度
Soil temperature土壤湿度
Soil moisture生长季Growing season 对照Ambient 21.49 ±0.13b 32.54 ±2.28a 增温Warming 2.40±0.09a 2.98 ± 0.90a 非生长季Ungrowing season 对照Ambient 1.81 ±0.23b 12.65 ± 1.03a 增温Warming 4.05 ± 0.35a 10.39 ± 1.23a (不同字母表示在0.05水平上显著差异)
(The different letters refer to significant effects at p < 0.05)Table 4. Results of t-test on soil moisture and temperature
-
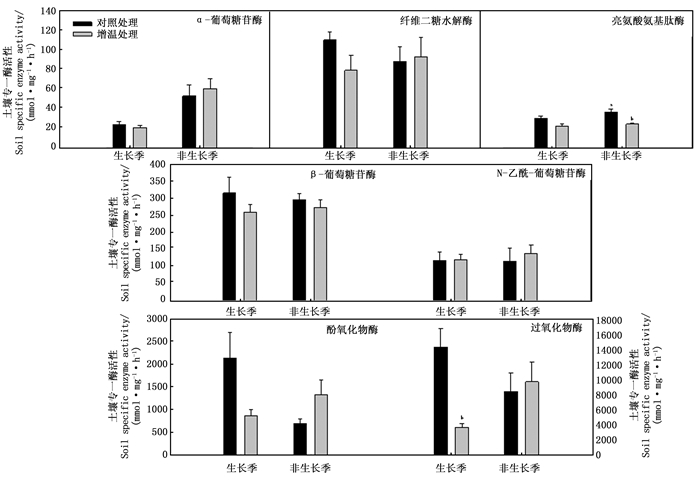
增温处理改变了土壤专一水解酶和氧化酶活性(图 1)。除NAG外, 生长季增温处理降低了AG、BG、CB和LAP专一酶活性, 但均未达到显著水平; 增温处理显著降低了PER活性, 未显著改变POX活性。非生长季增温处理增加了AG、CB、NAG、PER和POX专一酶活性, 但降低了BG和LAP专一酶活性, 且对LAP影响达到显著水平; 与生长季不同, 增温处理在非生长季不同程度地增加土壤专一酶活性, 但未达到显著水平。图 1和表 5可知: 除AG和LAP外, 对照处理非生长季酶活性均低于生长季; 增温处理非生长季酶活性均高于生长季, 且非生长季AG活性显著高于生长季活性(P=0.019)。增温处理在生长季普遍降低了土壤专一酶活性, 但非生长季增温处理则增加了土壤专一酶活性。说明土壤酶活性存在季节间变异, 且增温处理在不同季节间对土壤酶的影响不同。
处理Treatment AG BG CB NAG LAP P0X PER 对照Ambient 0.060 0.731 0.277 0.965 0.163 0.066 0.163 增温Warming 0.019 0.677 0.597 0.606 0.411 0.247 0.086 处理Treatment 水热比S/T 溶解性有机碳D0C/ (mg · g-1) 微生物量碳MBC/ (mg · kg-1) 微生物量氮MBN/ (mg · kg-1) 微生物碳氮比MBC/MBN 硝态氮N03-N/(mg · kg-1) 铵态氮NH4-N/(mg · kg-1) 对照Ambient < 0.01 0.150 0.556 0.100 < 0.01 0.634 < 0.01 增温Warming 0.037 0.013 < 0.01 0.027 0.057 0.156 0 044 (加粗字体表示在0.05水平上显著差异)
(The bold numbers refer to significant effects at p < 0.05)Table 5. Results of t-test on soil labile carbon and nitrogen pools and specific enzyme activity in growing and ungrowing seasons (p value)
-
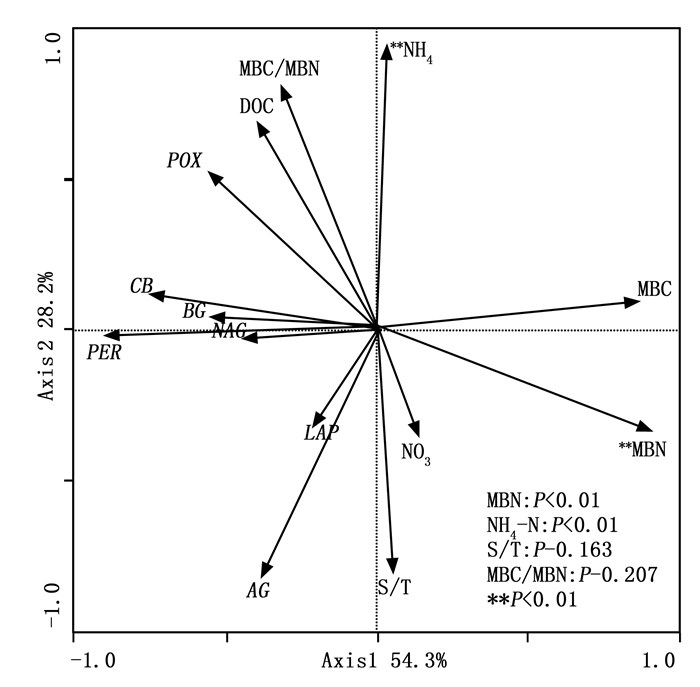
冗余度分析(RDA)表明: 9个环境因子变量中, 有2个环境因子(NH4-N和MBN)与专一土壤酶活性显著相关。RDA分析中第一主成分和第二主成分共可以解释9个环境因子与7种土壤专一酶活性关系变异的82.5%(图 2)。NH4-N和MBN共可以解释土壤专一酶活性变异的59.0%。RDA排序图说明主要环境因子和土壤专一酶活性之间的特异关系。其中LAP和AG与S/T和NO3-N呈正相关关系, POX与NH4-N、DOC和MBC/MBN呈正相关关系, NAG、PER、BG和CB则与DOC和MBC/MBN呈正相关关系。说明土壤专一酶活性变异受多种环境因子同时调控。
3.1. 样地土壤基本理化性质
3.2. 增温处理对土壤环境和活性碳库与氮库的影响
3.3. 增温处理对土壤酶活性的影响
3.4. 土壤酶活性的环境解析
-
本研究可知: 与对照样地相比, 增温处理土壤有机碳含量显著降低12.15%。与POST[29]和Shaver[30]等人的研究结果一致, 认为在气候变化背景下, 土壤有机碳含量可能会随着温度的升高而呈现下降的趋势。作为土壤碳库和氮库的最重要组成, 土壤有机碳和总氮的微弱变化都可能会对大气温室气体浓度产生显著的影响, 进而影响全球尺度的碳氮循环。增温处理土壤全氮含量低于对照处理, 但并未达到显著水平。与Rui等人[31]在高山草甸开展的增温控制试验得出的结论一致, 增温处理导致0~10 cm土壤总氮含量下降。增温处理虽导致土壤有机碳和总氮含量下降, 但并未引起土壤碳氮比显著变化。
作为土壤碳库和氮库中重要组成部分, 活性碳库和氮库(微生物量碳、微生物量氮、溶解性有机碳和溶解性有机氮)虽然所占比例不高, 却在调节土壤碳氮循环中发挥着重要的作用。研究表明: 生长季增温处理显著提高了土壤中微生物量碳氮, 但非生长季增温处理对微生物量碳氮的影响并不显著, 说明微生物量碳氮对增温处理的响应受季节变化影响。Xu等人[32]开展增温试验也发现土壤活性碳库和氮库对增温处理的响应受季节变化影响显著。溶解性有机碳作为土壤微生物有机体可直接利用碳源, 增温处理并未显著增加溶解性有机碳含量。Luo等人[33]研究表明, 温度和湿度并不是影响土壤溶解性有机碳的主要因子, 地下生物量等生物因子是调控土壤溶解性有机碳的重要因子。故增温处理虽显著增加土壤温度, 但并不能增加土壤中溶解性有机碳的含量。微生物量碳氮比在粗略水平上可以看作微生物中细菌和真菌的相对丰度比[34]。生长季中增温处理显著降低了土壤微生物碳氮比, 说明与对照样地相比增温样地的细菌群落占优势。已有整合分析研究表明[35]: 增温处理会显著增加土壤净氮矿化作用和净硝化作用。本研究观察到增温处理虽导致土壤硝态氮和铵态氮(生长季除外)含量增加, 但并未达到显著水平。这可能是因为增温处理导致细根生物量和土壤微生物量的增加促进了二者对土壤中无机氮的吸收和利用。
-
土壤酶作为土壤微生物活性和土壤理化性质的指示因子[11], 在调控土壤碳氮循环中发挥着重要的作用。由于土壤酶活性与土壤性质、微生物特性等密切相关, 所以增温处理下土壤酶活性可能出现季节性变化。在考察土壤酶活性的研究中, 专一酶活性可以把微生物和土壤酶活性建立较好的联系。图 1表明: 生长季中除NAG外, 增温处理不同程度降低其他4种水解酶专一酶活性, 但均未达到显著水平。说明气候变暖情况下, 酶催化反应变得更有效, 单位微生物产生的酶量降低。黄雪蔓[36]研究发现: 土壤微生物群落受土壤底物的数量(有机碳含量)和质量(碳氮比)影响。作为土壤微生物两大主要类群, 通常认为细菌与水解酶活性相关, 主要利用土壤中活性底物; 真菌与氧化酶活性相关, 主要利用土壤中惰性底物[37-38]。微生物量碳氮比作为粗略反映微生物群落中细菌和真菌相对丰度的指标, 且真菌比细菌具有更高的碳氮比, 所以微生物量碳氮比升高反映真菌相对丰富度更高。生长季中对照样地微生物量碳氮比(7.53)显著高于增温样地微生物碳氮比(6.58), 说明对照样地中真菌相对丰富度比增温样地的高, 真菌通常分泌氧化酶以分解大分子化合物, 故生长季对照样地内过氧化物酶活性显著高于增温样地的。
环境因子变化可影响土壤底物扩散作用[39]和底物利用有效性[40]。非生长季中土壤温度因子成为限制性因子时, 底物扩散作用和有效性受到限制, 微生物利用底物效率和微生物活性也随之降低。增温处理显著提高了非生长季土壤温度, 故非生长季增温样地微生物活性增强, 微生物为满足自身生长需要, 单位微生物量会产生更多的酶以提高其对土壤养分的利用, 所以增温处理在非生长季对土壤专一酶活性多表现出促进作用。
-
除温度因子外, 土壤湿度也影响土壤底物的扩散作用[39], 作者引入水热比(S/T), 考察温湿度因子共同作用对土壤专一酶活性的影响。图 2可知: LAP和AG与S/T和NO3-N显著正相关, 而温度因子对氮循环酶LAP的作用不明显[41], 说明LAP活性受土壤湿度影响, 该结论与Brzostek通过整合分析发现LAP酶活性受土壤湿度限制的结论一致[3]; 表 5可知: 增温样地和对照样地非生长季土壤NO3-N与生长季土壤NO3-N差异并不显著, 但非生长季S/T显著高于生长季, 故非生长季LAP和AG活性与生长季相比均表现出增加趋势(图 1, 表 5)。RDA排序图表明NAG、BG和CB则与DOC和MBC/MBN呈正相关关系。表 5可知: 非生长季与生长季相比, 对照样地MBC/MBN显著降低是引起非生长季对照样地酶(NAG、BG和CB)活性低于生长季的主要原因, 说明微生物群落结构的变化可能是影响土壤专一酶活性季节变化的主要原因。You[42]在该区域研究也发现微生物群落结构与土壤酶活性之间具有很强的相关性, 氧化酶活性与真菌相关而细菌与水解酶活性相关。这与我们得到的结论较为一致。图 2可知, POX与NO3-N呈负相关关系; PER与NO3-N和NH4-N也呈负相关, 与Sinsabaugh等人[37]研究结果一致, 认为氧化酶活性与土壤无机氮可利用性呈负相关关系。我们把MBC作为分子将总土壤酶活性专一化, 故RDA分析中土壤专一酶活性与MBC和MBN呈负相关关系。
4.1. 增温处理对土壤碳库和氮库的影响
4.2. 增温处理对土壤专一酶活性的影响
4.3. 环境因子变异对专一土壤酶活性的影响
-
(1) 增温处理显著提高了土壤温度, 但并未显著降低土壤湿度。
(2) 生长季增温处理引起土壤温度增加改变了酶催化反应效率进而改变了土壤专一酶活性; 除PER外, 增温处理导致土壤专一酶活性的降低并未达到显著水平。非生长季增温处理普遍增加了土壤专一酶活性, 说明土壤专一酶活性对增温处理的季节性响应不同。
(3) 除微生物量氮和铵态氮外, 其他环境因子, 诸如: 土壤水热比、微生物量碳、微生物量碳氮比、溶解性有机碳和硝态氮等是影响土壤专一酶活性在不同处理间和季节间变异的主要原因。







 DownLoad:
DownLoad: