-
松树枯萎病的流行与危害已导致世界森林生态系统产生了严重危机[1]。对松枯萎病的发生与流行机制的研究仍在开展与不断的深入。自从日本报道松材线虫(Bursaphelenchus xylophilus) 能引起松树枯萎后,松材线虫已被认为是松材线虫病的病原[2-3],我国把松树枯萎病称为松材线虫病。但是随着研究的深入,发现单纯地仅以松材线虫为病原还不能解释自然界复杂多样的松树枯死现象,可能是一种(或一类)松材线虫的伴生菌参与了致死松树或产生毒素导致松树枯萎[4-11]。进入本世纪后, 有关枯萎松树内松材线虫的伴生细菌种类和病理作用及与松材线虫的关系研究报道越来越多, 拓展和深化了松树枯萎病研究范围和层次[12-15]。但现在面临的问题是,随着分离鉴定的细菌种类越来越多[16-18],要确定究竟是哪种细菌与松材线虫最为密切,哪种细菌具有真正病原作用参与了松树枯死的过程等问题,需要深入研究。荧光假单胞杆菌(Pseudomonas fluorescens)是众多枯死松树内细菌中鉴定到种研究最多的菌种,但报道的只是在实验室得到的可引起黑松(Pinus thunbergii Parl.)苗木或细胞枯萎的一些结果[19-22],没有在林间进行调查来证实荧光假单胞杆菌协同松材线虫促使了松树枯萎。而且荧光假单胞杆菌是一种动物病原细菌,也是一种能抑制或拮抗植物病原细菌的生物防治细菌[23]。很少有动物病原细菌也能同时成为植物病原细菌的,这是因为动物病原细菌不能分泌产生纤维素酶,植物细胞的细胞壁对动物病原细菌具有天然的免疫性。松材线虫的耐久性幼虫(LⅣ幼虫)是侵入松树的第一虫态,也是离开松树的最终虫态[24]。LⅣ幼虫体上是否携带细菌,带的是何种细菌十分值得研究。因为LⅣ幼虫如携带细菌,表明这种细菌与松材线虫的关系密切,同时与松材线虫、松褐天牛一起参与了松树枯萎的全过程。在LⅣ侵入松树时,携带的细菌也直接进入了松树;在LⅣ离开松树时,这种细菌也被携带离开松树,该种细菌最有可能就是引起松树枯萎的病原细菌。按这个思路,作者开展了从松材线虫LⅣ幼虫体上分离鉴定细菌的研究,获得了香茅醇假单胞杆菌(Pseudomonas citronellolis Seubert),现将研究结果报道如下。
HTML
-
2012年9月上旬作者在宁波市余姚市和慈溪市松材线虫病发生流行松林内锯取当年枯死的马尾松(Pinus massoniana Lamb.)和黑松(P. thunbergii Parl.)树干数段,置室内养虫笼内。2013年5月中旬起随时捕获羽化出来的松褐天牛成虫,置实体显微镜下解剖松褐天牛成虫,见到有线虫析出,用细针挑出线虫到载玻片上,制成临时玻片,在显微镜下检查确认为松材线虫的LⅣ幼虫后,再仔细观察幼虫的外部形态特征及携带微生物情况。
-
把野外采集的新鲜松针清洗晾干,用医用酒精擦针叶表面消毒后,横向剪切成长12 mm的松针段,再用手术刀纵切松针成细丝备用;用镊子把备用的松针丝移到载玻片中央,围成一个正方形后,中间加入1滴蒸馏水;用细针把解剖所得的LⅣ幼虫挑到载玻片上,每片挑入LⅣ幼虫5条,盖上盖玻片,制成LⅣ幼虫携带细菌的微量增殖玻片。制作这样的玻片5片,然后置28℃恒温箱保湿培养12 h。之后,把玻片置在显微镜下定性观察细菌生长情况。细菌数量在400X镜下定性计数,分5级:大量+++,表示镜下细菌密集,似沙漠状; 中量++,表示镜下细菌紧挨,均匀分布; 少量+,表示镜下细菌稀疏;偶见+/-,表示偶尔见有细菌;无-,表示镜下检测不到细菌。
-
用接种环蘸经过微量增殖的菌悬液,在NA平板上划线分离,经28℃培养箱中培养3 d,挑取单菌落纯化,得到的菌株为14个(分别予以编号)。
-
利用TaKaRa MiniBEST Bacterial Genomic DNA Extraction Kit提取细菌基因组DNA。
-
采用细菌16S rDNA扩增的通用引物[25-26]。正向引物:5′-AGA GTT TGA TCA TGG CTC AG-3′;反向引物:5′-ACG GTT ACC TTG TTA CGA CTT-3′,由南京金斯瑞生物技术有限公司合成。PCR反应体系(25 μL):10×buffer 2 μL,dNTP(10 mmol·L-1)1 μL, MgCl2 (25 mmol·L-1)1.5 μL,正向引物(10 μmol·L-1)和反向引物(10 μmol·L-1)各2 μL,TaqDNA聚合酶(5 U·μL-1)0.2 μL, ddH2O 15.3 μL, 模板DNA 1 μL。循环条件:94℃预变性4 min,94℃变性30 s,54℃退火30 s,72℃延伸2 min,30次循环;最后72℃延伸7 min。PCR产物经1%琼脂糖凝胶电泳检测后,在-20℃下保存备用。PCR产物的纯化和测序由南京金斯瑞生物技术有限公司完成。
-
测序后的序列经校正后,与EzTaxon-e server (http://eztaxon-e.ezbiocloud.net/)中16S rRNA核酸数据进行BLAST比对分析,比较测试菌株同现有数据库中相应序列的相似程度。利用ClustalX 1.8.1进行序列比对,MEGA 5.03软件选用Kimura2-parameter距离模型进行Neighbor-joining分析生成系统发育树,发育树用自展(Bootstrap)分析法进行检验,共循环1 000次。
1.1. LⅣ幼虫分离
1.2. LⅣ幼虫携带细菌的微量增殖
1.3. LⅣ幼虫携带细菌的分离培养
1.4. LⅣ幼虫携带细菌16S rDNA序列分析
1.4.1. 基因组DNA的提取
1.4.2. 16S rDNA PCR扩增
1.5. 数据分析
-
解剖镜检松褐天牛成虫35头,其中18头成虫体内检到LⅣ幼虫,占5.14%。LⅣ幼虫少量分布在松褐天牛成虫体表,气管内也能检到较多LⅣ幼虫,生殖器内LⅣ幼虫数量最多。本次分离细菌用的LⅣ幼虫从松褐天牛雌虫生殖道内解剖得到(图 1-a,b,c)。LⅣ幼虫的形态特征为:头顶圆丘形,口针、食道和食道球退化不明显(图 4);尾部带有尾尖突(图 1-e);体长L 0.60.7 mm,体宽a 0.0300.035 mm; 虫体表面覆盖有厚厚的粘液,显得较胖,内部结构不透明(图 1-f);细菌携带率100%;每条LⅣ携带线虫量在1.4×1054.5×105。
-
经玻片培养,细菌数量快速增长,12 h后在400X显微镜下能见到像“沙漠似”的细菌群落(图 1-g),也可见到LⅣ幼虫在细菌群落中缓慢蠕动(图 1-h);在1 000X下能见到杆形、长径1.11.5 μm,短径0.40.5 μm细菌颗粒(图 1-i)。这种细菌常2颗串在一起,做顺时针或逆时针旋转或向前运动,这表明这种菌有鞭毛;也有单颗的,在镜下振动和移动[27]。根据形态特征和镜下运动方式,表明这种细菌是一种假单胞杆菌(Pseudomonas sp.)。
-
假单胞杆菌菌株14菌体直短杆状,大小0.40.5×1.11.5 μm,革兰氏染色阴性,在NA培养基上菌落圆形,暗白色,边缘整齐,低凸起。
-
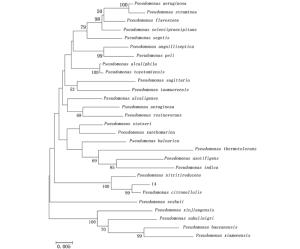
菌株14的16S rDNA序列全长1 448 bp,将该序列与EzTaxon-e中相关数据进行相似性分析,菌株14和Pseudomonas citronellolis DSM 50332(Z76659)相似性达到99%。并选取27个菌株的16S rDNA序列,通过MEGA 5.03软件选用Kimura2-parameter距离模型进行Neighbor-joining分析生成系统发育树(图 3)。系统发育分析表明,在与菌株14具有较高同源性的假单胞菌属细菌中,其和Pseudomonas citronellolis DSM 50332(Z76659)处于同一分支,表明菌株14与Pseudomonas citronellolis的亲缘关系最近。
2.1. 松材线虫LⅣ幼虫的形态
2.2. LⅣ幼虫携带的细菌增殖
2.3. 菌株形态及培养性状
2.4. LⅣ幼虫携带细菌的16S rDNA序列分析
-
松树枯萎病病死木内除能检到病原松材线虫外,还能检到其它数量庞大的寄生虫和微生物群落[28]。这些微生物群落中,细菌的种类和病原性及与松材线虫的关系是目前研究的重点和热点[4, 8, 12-13]。随着研究的深入,从枯死松木中分离所得的细菌种类越来越多,由此衍生的与松材线虫的关系也越来越复杂,如何理清这些错综复杂的关系是目前摆在植物病理学者面前的难题。笔者从松材线虫LⅣ幼虫上分离得到纯净单一的细菌——香茅醇假单胞杆菌(Pseudomonas citronellolis),为破解这些复杂关系提供了新的思路。LⅣ是松材线虫只存在于松褐天牛成虫体内的一个最特殊的虫态,是侵入松树和离开松树的唯一虫态[29]。这个虫态携带细菌,表明松树—细菌—线虫—松褐天牛,是一个整体,构成一个完整的循环。在春夏季松褐天牛成虫携带LⅣ和细菌离开枯死松树,飞向健康松树补充营养时,携带的LⅣ和细菌侵入松树,细菌和线虫在健康松树内大量增殖,致死松树;次年春夏季待松褐天牛化蛹羽化为成虫时,线虫又带着细菌在松褐天牛体内集中,松褐天牛羽化出孔形成新的侵染循环。从这点推测,这种细菌必须是纯净的、对昆虫和线虫不具致病性;线虫、细菌、松褐天牛之间的关系是紧密的、稳固的、长期进化所形成的。如果是多个细菌种类就有可能存在对昆虫有致病性的细菌被LⅣ带入昆虫体内,导致宿主昆虫感病,那么自然界就不可能存在这个侵染循环系统。
-
笔者从松材线虫LⅣ幼虫体上分离得到的细菌是香茅醇假单胞杆菌,在有关松材线虫与细菌的文献中尚未见报道。这个菌种很值得进一步深入研究,因为它有可能是真正引起松树枯萎病的病原。首先,香茅醇假单胞杆菌是从松林土壤分离得到的细菌[30],所以很有可能该细菌来于松树林地的枯枝落叶中,即与松树的关系密切;第二,该种细菌能产出大量高效的纤维素降解酶[31],表明该菌能破解植物细胞壁屏障。植物细胞壁由纤维素组成,能成为树木病原的微生物必定能大量产生纤维素酶,溶解细胞壁后,致病细菌才能从植物体内吸收营养生长[32],松材线虫也能分泌纤维素酶,许多学者认为松材线虫分泌的纤维素酶就是松树枯萎病致病物质[33-35]。香茅醇假单胞杆菌与LⅣ幼虫共生,提示促使松树枯萎的纤维素酶主要是该菌所产生的;第三,该菌能氧化萜烯类化合物和降解多种有机烃链的能力[36-39], 松树树脂道内充满了萜烯物质[40],是松树分泌的免疫体液,该菌如进入树脂道氧化萜烯类化合物和降解多种有机烃, 可能会对松树的免疫系统起到破坏作用;第四, 该菌能合成多种有机化合物[41-42],提示进入松树后有可能合成对松树有毒的物质造成松树枯萎;第五,该菌能分泌羧化酶,氧化丙酮酸[36],丙酮酸是植物光合作用和呼吸作用的重要中间产物,提示香茅醇假单胞杆菌进入松树体内后可能会干扰光合作用和呼吸作用。香茅假单胞杆菌的这些特殊功能,为揭示松树枯萎机制提供了一种新的病原和思路,也可能该菌就是引起松树枯萎的重要病原。这些结论值得今后进一步深入研究和证实。







 DownLoad:
DownLoad: