-
土壤盐碱化已经成为人类面临的主要生态危机,中国盐碱土地资源总额约9 913×104 hm2[1]。盐碱胁迫会对植物产生诸多不利的影响,包括碱性盐造成的渗透胁迫[2]、过量的活性氧积累和光合作用下降[3],此外,高pH还会增加根际Na+的吸收,增强细胞内Na+的毒害效应[4-5],影响离子对的形成并沉淀金属离子,造成严重的养分胁迫[6],从而影响植物的生长发育,使盐碱地难于建立植被,严重制约了城市景观建设。城市园林绿化是城市建设不可缺少的一部分,而盐碱地绿化是城市绿化中的一个难点。素有“花中西施”的杜鹃花作为世界四大花卉之一,因其具有株型丰富、开花繁茂的特点,成为园林绿化、庭院美化不可或缺的花灌木。然而,杜鹃花属于典型的喜酸性植物,在土壤pH为46时生长良好,稍遇弱碱,叶片就会焦枯脱落,以致植株停止生长而死亡。我国不少城市土壤呈中性偏碱或者碱性,杜鹃花很难实现露地栽培[7]。日益加剧的土壤盐碱化严重限制了杜鹃花的大量推广应用。因此,研究杜鹃耐盐碱的生理途径具有重要的意义。
β-氨基丁酸(β-aminobutyric acid,BABA)是一种非蛋白质氨基酸,在自然界中稀少存在[8]。一直以来,BABA在提高植物抗病能力中得到广泛研究[9]。最近的研究表明,它在诱导植物抵御非生物胁迫中也发挥着重要作用。外源BABA可以缓解Cd胁迫下菊花叶片丙二醛(MDA)含量的增加和相对电导率的升高,促进Cd胁迫下根系对Cd的吸收及其向地上部的转运,从而改善Cd胁迫下菊花的生长[10]。BABA还能诱导植物适应盐胁迫[11]。大麦种子经BABA处理过后,可以增加盐胁迫下种子的鲜质量、干质量和相对含水量,上调抗氧化蛋白和病程相关蛋白,增强大麦对盐胁迫的耐受性[12]。在调控植物适应高温、干旱和低钾胁迫等方面[13-15],BABA也发挥着重要作用。但关于BABA调控植物缓解NaHCO3胁迫的研究尚未见报道,研究外源BABA对碱胁迫下杜鹃生长的影响,可为利用外源物质提高杜鹃的耐碱性奠定基础。
HTML
-
试验材料为杜鹃品种‘神州齐’,同一批次的2年生扦插苗,由四川省农业科学院园艺研究所提供。
-
盆栽试验于2016年7—9月在四川省成都市锦江区园艺所科研基地进行。2016年7月下旬选择生长健壮、长势基本一致的无病虫害植株,平均苗高29.2 cm,平均冠幅17.5 cm,从塑料营养袋中取出,剪除老叶,用清水洗净根系,剪去烂根,定植于上口直径18 cm,下口直径13 cm,高度18 cm的塑料营养袋中,每盆定植3株。用4 mol·L-1的盐酸将河沙浸泡5 h,再用蒸馏水反复浸洗至pH为7.0±0.2,去离子水冲洗3次后烘干,每盆装1.6 kg细沙。用适合杜鹃无土栽培的1/4营养液(pH5.5,EC1.2)进行培养[16],其中所含大量元素为:NH4NO3 240.1 mg·L-1、K2SO4 17.4 mg·L-1、KNO3 283.1 mg·L-1、KH2PO4 136.1 mg·L-1、K2HPO4 17.4 mg·L-1、MgSO4·7H2O 147.9 mg·L-1、Ca(NO3)2·4H2O 614.0 mg·L-1、NaCl 701.3 mg·L-1、Na2·EDTA 37.3 mg·L-1、FeSO4·7H2O 27.8 mg·L-1。每3 d浇灌1次,每次500 mL。
2016年8月6日,待植株缓苗后开始进行BABA处理(表 1),先将BABA溶于1/4营养液中进行浇灌,处理3 d后进行NaHCO3胁迫处理,参照萨如拉[17]、刘建新[18]等人的方法,NaHCO3处理液以每天递增10 mmol·L-1至50 mmol·L-1,然后每次以含50 mmol·L-1NaHCO3的营养液浇灌,浇灌量为细沙持水量的2倍,每3 d浇1次。每处理4盆,每盆3株,试验重复3次。
编号
Serial number试验设计
Experimental designCK 1/4营养液 T1 50 mmol·L-1NaHCO3+1/4营养液 T2 50 μmol·L-1BABA+50 mmol·L-1NaHCO3+1/4营养液 T3 100 μmol·L-1BABA+50 mmol·L-1NaHCO3+1/4营养液 T4 200 μmol·L-1BABA+50 mmol·L-1NaHCO3+1/4营养液 T5 400 μmol·L-1BABA+50 mmol·L-1NaHCO3+1/4营养液 注:BABA处理3d后再进行NaHCO3胁迫处理。
Note:plants were pretreated with water or BABA for 3 days,and then growth with or without NaHCO3.Table 1. The factors and levels of this experiment
胁迫处理14 d后取杜鹃枝条上第46片功能叶放入液氮中速冻后,于-80℃下保存,用于生理指标的测定。
-
2016年8月23日,每个处理12盆中随机选3盆,每盆取1株,用蒸馏水将植株上附着的杂质洗净,用吸水纸将植株表面的水分吸干,将植株分为地上、地下两部分,称鲜质量;在105℃烘箱内杀青30 min,再在75℃下烘干至恒质量,称取干质量。
-
于2016年8月20日—8月22日(晴朗无云)上午9:0011:00,每个处理随机选取9盆,每盆取1株,共9株植株,自顶端向下第46片功能叶,用Li-6400便携式光合仪测定叶片净光合速率(photosynthetic rate,Pn,μmolCO2·m-2·s-1)、蒸腾速率(transpiration rate,Tr,mmolH2O·m-2·s-1)、胞间二氧化碳浓度(intercellular CO2 concentration,Ci,μmolCO2·mol-1)、气孔导度(conductance to H2O,Gs,molH2Om-2·s-1)。测定时光照强度为(1 000±10)μmol·m-2·s-1,CO2浓度为(400±10)μmol·mol-1。光合色素的测定参照施海涛[19]的方法。
-
抗坏血酸过氧化物酶(APX)活性测定参照汤章城[20]的方法,超氧化物歧化酶(SOD)含量的测定参照Giannopolitis等[21]的方法,过氧化物酶(POD)活性的测定参照张志良和翟伟菁[22]的方法,过氧化氢酶(CAT)活性、过氧化氢(H2O2)和丙二醛(MDA)含量的测定参照李和生[23]的方法,脱氢抗坏血酸还原酶(DHAR)活性的测定参照Ma和Cheng[24]的方法,谷胱甘肽还原酶(GR)活性参照Milosevic[25]等人的方法测定。
-
利用Excel2007对试验数据进行初步计算,采用SPSS19.0软件进行单因素方差分析、Duncan’s多重比较分析,采用Excel2007软件作图。
1.1. 试验材料
1.2. 试验设计
1.3. 测定指标与测定方法
1.3.1. 生物量的测定
1.3.2. 光合参数和光合色素含量的测定
1.3.3. 生理指标的测定
1.4. 数据处理与统计分析
-
表 2显示,未经BABA预处理(T1处理)杜鹃植株的鲜质量和干质量显著降低,BABA预处理能显著增加杜鹃植株地上部鲜质量,而对地上部干质量、地下部鲜质量、干质量均无显著影响。未经BABA预处理的杜鹃植株地上部和地下部鲜质量较CK分别下降66.3%和42.0%,干质量较CK分别下降48.1%和34.1%。经BABA预处理后,随着处理浓度的增大,杜鹃植株各部分生物量先增大后减小,200 μmol·L-1BABA处理(T4处理)杜鹃植株地上部和地下部鲜质量、地上部干质量分别较T1处理增加了52.4%、36.6%和34.7%,100 μmol·L-1BABA处理(T4处理)杜鹃植株地下部干质量较T1处理增加了23.8%。可见,在NaHCO3胁迫下,添加外源BABA可以缓解碱胁迫对植株生长的抑制。
处理
Trentment生物量Biomass/(g·plant-1) 地上部鲜质量
Shoot fresh weight地下部鲜质量
Root fresh weight地上部干质量
Shoot dry weight地下部干质量
Root dry weightCK 8.92±1.01a 4.00±0.65a 3.16±0.54a 3.31±1.14a T1 3.01±0.97c 2.32±1.86a 1.64±0.44b 2.18±0.38a T2 4.98±1.08bc 4.46±1.12a 2.09±0.45ab 2.75±0.94a T3 4.28±1.90bc 2.25±2.00a 1.46±0.66b 2.86±0.81a T4 6.33±1.68ab 3.66±0.76a 2.51±0.74ab 2.36±0.80a T5 4.4 ±2.32bc 2.03±1.04a 2.19±0.89ab 2.73±1.38a 注:表中数据为平均值±标准差。同列不同字小写母表示差异显著(P<0.05),下同。
Note: Different lowercase letters indicated significant difference at 0.05 level,the same as below.Table 2. Effects of BABA treatment with different concentrations on biomass in leaves of Rhododendron 'shen zhou qi'under NaHCO3 stress
-
由表 3可看出,NaHCO3胁迫下,杜鹃叶片Pn、Tr和Gs显著降低,Ci显著升高,BABA预处理后,Pn显著升高,Ci显著降低,Tr和Gs无显著变化。杜鹃叶片Pn和Tr随BABA处理浓度的增加先增大后减小,而Ci和Gs则相反。外源BABA显著缓解碱胁迫对Pn的限制,T3处理达到最大值,是T1处理的2.3倍;不同浓度的BABA能缓解NaHCO3胁迫对Tr的限制,其中,以T4处理效果最好,较T1处理增加了39.6%;Ci随外源BABA浓度的增大先降低后升高,在T4处理达到最低值,比T1处理减少了64.8%;Gs在T3处理时达到最小值,比T1处理减小了58.8%。
处理
Treatment净光合速率
Pn(μmol·m-2·s-1)蒸腾速率
Tr/(mmol·m-2·s-1)胞间CO2浓度
Ci(μmol·mol-1)气孔导度
Gs/(mol·m-2·s-1)CK 3.60±0.50a 1.63±0.66a 139.85±47.41b 0.04±0.015a T1 1.51±0.73c 0.48±0.37b 435.20±112.45a 0.01±0.010b T2 3.02±0.76ab 0.56±0.28b 205.00±108.80b 0.02±0.006b T3 3.52±0.44a 0.51±0.29b 180.12±42.77b 0.01±0.008b T4 2.24±1.11bc 0.67±0.36b 153.40±84.29b 0.01±0.009b T5 1.62±1.06c 0.62±0.20b 179.69±134.49b 0.02±0.006b Table 3. Effects of BABA treatment with different concentrations on gas exchange parameters in leaves of Rhododendron 'shen zhou qi'under NaHCO3 stress
从表 4可以看出,碱胁迫下,杜鹃叶片中叶绿素a、叶绿素b、类胡萝卜素和总叶绿素含量均显著降低;外源BABA可以显著增加碱胁迫下叶绿素b、类胡萝卜素和总叶绿素的含量,但对叶绿素a的含量无显著影响。NaHCO3胁迫下,杜鹃叶片中叶绿素a、叶绿素b、类胡萝卜素和总叶绿素含量分别比对照降低了52.7%、61.3%、74.3%和55.5%;外源BABA可以略微增加NaHCO3胁迫下这些色素的含量,随着BABA浓度的增大,杜鹃叶片叶绿素a和总叶绿素含量先升高后降低,T4处理最高,分别是T1处理的1.8和1.9倍;而杜鹃叶片叶绿素b和类胡萝卜素含量则随着BABA浓度的增大先略微降低再升高,分别在T5和T4处理时达到最大值,分别是T1处理的2.1和2.7倍,但仍低于CK,说明BABA对NaHCO3胁迫下杜鹃叶片类胡萝卜素含量的减少有较好的缓解作用,而对叶绿素效果不明显。
处理
Treatment叶绿素a含量
Chl a content/(mg·g-1)叶绿素b含量
Chl b content/(mg·g-1)类胡萝卜素含量
Caro content/(mg·g-1)总叶绿素含量
Chl Content/(mg·g-1)CK 2.07±0.23a 0.75±0.26a 0.74±0.13a 2.83±0.41a T1 0.98±0.43b 0.29±0.02c 0.19±0.07c 1.26±0.41b T2 1.47±0.3ab 0.44±0.14bc 0.52±0.19ab 1.92±0.43ab T3 1.51±0.49ab 0.41±0.12bc 0.44±0.04b 1.92±0.45ab T4 1.80±0.53ab 0.60±0.14ab 0.52±0.13ab 2.40±0.64a T5 1.67±0.53ab 0.61±0.10ab 0.47±0.15b 2.29±0.58a Table 4. Effects of BABA treatment with different concentrations on photosynthetic pigment contents in leaves of Rhododendron 'shen zhou qi' under NaHCO3 stress
-
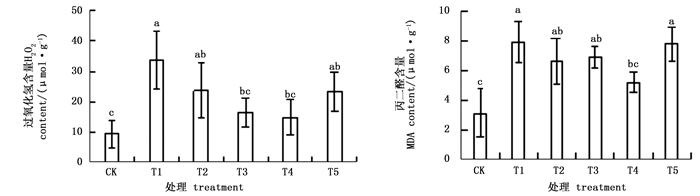
图 1表明:杜鹃叶片H2O2和MDA含量在NaHCO3胁迫后均显著升高,且两者的含量在BABA预处理后显著降低。NaHCO3胁迫下,H2O2和MDA含量分别是CK的3.6和2.6倍。不同浓度的外源BABA处理可以降低杜鹃叶片H2O2的积累,缓解NaHCO3诱导的膜质过氧化,使MDA的积累量显著降低。随着BABA浓度的增大,杜鹃叶片H2O2含量先降低后升高,4种浓度BABA处理下,200 μmol·L-1BABA处理的H2O2含量最低,仅为T1处理的44.0%;杜鹃叶片MDA含量随外源BABA浓度的增大先略微上升后下降,继而又上升,T4处理杜鹃叶片MDA含量比T1处理减少了34.1%。
-
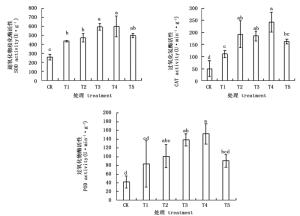
图 2表明,NaHCO3胁迫下杜鹃叶片SOD、CAT和POD活性均显著上升,外源BABA可显著增加NaHCO3胁迫下这些酶的活性,其中以200 μmol·L-1效果最好。经不同浓度的外源BABA预处理后,SOD、CAT和POD活性的变化趋势较一致,都是随BABA浓度的增大先上升后下降,在T4处理达到最大值,分别是T1处理的1.37、2.18和1.83倍。
-
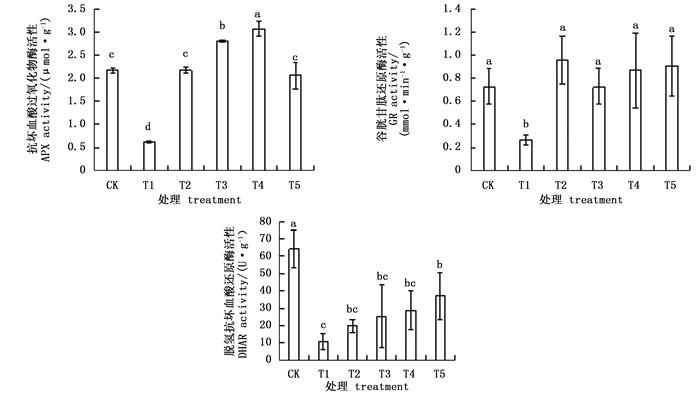
图 3表明,APX、GR和DHAR活性在NaHCO3胁迫后均显著下降,而外源BABA显著增加了这些酶的活性。经不同浓度的外源BABA预处理后,T2-T5处理杜鹃叶片APX随BABA浓度的升高先上升后下降,其中,T4处理APX活性最高,是T1处理的4.98倍;GR活性随BABA浓度的增大先降低后升高,T2处理GR活性最高,是T1处理的3.6倍;DHAR活性随BABA浓度的增大一直升高,在T5处理时DHAR活性最高,是T1处理的3.4倍。
2.1. 外源BABA对NaHCO3胁迫下杜鹃花生物量的影响
2.2. 外源BABA对NaHCO3胁迫下杜鹃光合特性和光合色素的影响
2.3. 外源BABA对NaHCO3胁迫下杜鹃叶片H2O2和MDA含量的影响
2.4. 外源BABA对NaHCO3胁迫下杜鹃叶片SOD、CAT、POD活性的影响
2.5. 外源BABA对NaHCO3胁迫下杜鹃叶片AsA-GSH循环中酶活性的影响
-
BABA天然存在于植物中,并在胁迫后被诱导[26]。其通过增强拟南芥的防御反应从而增强对生物和非生物胁迫的抵抗能力。研究表明,BABA诱导胁迫诱导的形态发生反应(SIMR),且经BABA处理的拟南芥会积累胁迫信号调节剂-花青素[27]。BABA通过增强ABA依赖性信号通路和水杨酸依赖性防御机制起作用[28]。
非生物胁迫下,植物的个体形态发育受到显著影响,其中生长抑制是植物对逆境响应最敏感的过程[29]。50 mmol·L-1的NaHCO3胁迫下杜鹃叶片失绿黄化,植株光合作用下降,生物量减少,与前人报道NaHCO3胁迫下杨树[30]、黑麦草[31]的生理效应相同。Yang和Fang等人认为可能是由于NaHCO3胁迫下Na+的大量增加破坏了离子平衡,根际高pH环境影响了溶液中离子的活性,导致金属离子沉淀而失去有效性[2, 6],其中受影响较为严重的是Fe、Mn、Mg、Ca等[32],Fe和Mg又是合成叶绿素必需的元素,这可能是导致NaHCO3胁迫下杜鹃叶片叶绿素含量减少,净光合速率下降的重要原因。本研究中,外源BABA减小了NaHCO3胁迫下杜鹃叶片叶绿素含量的下降幅度,Liu[33]等研究发现,BABA对酸雨造成的拟南芥叶片叶绿素含量的降低有显著的增加作用;施旭丽[15]等人的研究结果也表明,BABA对Cd胁迫下菊花叶片叶绿素的降解具有保护作用,本研究结果与之一致。
BABA有诱导气孔关闭的功能,气孔孔径大小的快速降低和ABA的积累是BABA诱导植物耐旱耐盐性的基础。研究表明,BABA通过在植物体内积累ABA从而诱导气孔关闭以及下游抗逆基因的表达来提高拟南芥的耐盐性和抗旱性[33],相关研究表明,BABA预处理可以增加植物体内Ca2+含量[34]。本研究中,100200 μmol·L-1外源BABA预处理增强了NaHCO3胁迫导致的杜鹃叶片Gs下降,同时Pn显著提高。100200 μmol·L-1BABA诱导ABA的快速产生,促使保卫细胞内Ca2+浓度的升高,活化阴离子通道,从而促使阴离子流出,并使膜电势向负值小的方向偏移,这种膜的去极化形成一个有利于K+通过一种外向的K+通道而被动外流的化学梯度,从而促进气孔关闭,减小Gs,提高杜鹃的水分利用率,缓解渗透胁迫[35-36]。盐胁迫下,植物响应土壤水势的不平衡快速吸收离子,不断积累的Na+和Cl-具有破坏新陈代谢的毒害作用,Jakab等人认为BABA可能通过诱导ABA降低蒸腾速率来缓解离子的毒害作用[15]。由于相关报道较少,关于BABA诱导杜鹃缓解NaHCO3胁迫的生理机制仍需进一步深入探讨。
ROS可对植物产生一系列的有害作用,引起膜脂质过氧化过程增强,造成对生物膜的破坏。植物组织的氧化损伤通过酶和非酶抗氧化代谢的协同作用减轻。这个系统包括β-胡萝卜素,抗坏血酸(AA),α-生育酚,还原型谷胱甘肽(GSH)和酶,包括SOD,PDD,APX,CAT,PPO和GR等[37]。前人已经在西红柿[38]、山黧豆[39]、南蛇藤[40]等植物中证明了增强抗氧化酶活性与增强植物抗NaHCO3胁迫能力之间的关系。本试验中,NaHCO3胁迫造成杜鹃叶片内产生大量的ROS,并引起膜脂的过氧化反应。研究表明,BABA可以导致初级代谢的改变,同时激活抗氧化系统和水杨酸、茉莉酸、脱落酸信号通路[41]。BABA能通过上调抗氧化蛋白(SOD、CAT、POD)的表达激活抗氧化防御系统[12, 42]。表明外源BABA有效诱导保护酶系统也成为其有效缓解逆境胁迫的重要机制之一。SOD将O2-歧化为H2O2,而CAT、APX、GR负责清除H2O2[43]。本试验中,BABA处理增强了杜鹃叶片SOD、POD、CAT、APX和DHAR活性,以加速ASA-GSH循环,消除生成的过度有害的氧化物基团。缓解NaHCO3胁迫造成的膜脂质氧化损伤,这与Du等[44]人的研究结果一致,Cao等[45]人的研究结果也表明,BABA通过拟南芥中部分GSH依赖性信号通路缓解Cd胁迫,而Bacceli[11]等人则指出,BABA处理后ROS的平衡会根据植物和胁迫的种类受到不同的影响。
-
本研究结果表明,外源BABA处理提高了NaHCO3胁迫下杜鹃叶片的光合作用,并增强了抗氧化酶活性,有效缓解了NaHCO3胁迫下的膜脂过氧化,从而改善其生长状况,且100200 μmol·L-1的外源BABA处理效果最好。






 DownLoad:
DownLoad: