-
绝大多数木本竹子为多年生一次性开花植物,开花十分罕见[1-2],且由于雌、雄蕊大多异熟,所以即使在盛花期其授粉成功率也很低,种子获取十分困难[3-6]。因此,竹子传统的繁殖方式以分蘖、分株、埋鞭等方法为主,但这些方法存在母竹消耗多,繁殖系数低等问题[7]。为此,科技人员一直在探求高效率的繁育方法,其中,最有效的就是通过竹子组织培养获得再生植株。目前,竹子组织培养也是竹苗繁育、竹子生物技术、种质遗传改良等研究得以实现的重要技术基础,因此,竹子组织培养也得到了越来越多的关注[8]。竹子组织培养较一般植物困难,易污染、褐化率高、易玻璃化是其最常见的三大难题[9],而竹类外植体材料的消毒灭菌是其组织培养的首要环节。由于竹类外植体内生菌较多[9],特殊的中空结构以及其表皮细胞具有致密的乳突和较厚的角质层阻碍消毒液的渗透[10],造成竹类植物材料彻底消毒灭菌尤为困难。
云南甜龙竹(Dendrocalamus brandisii (Munro) Kurz)和黄竹(D. membranaceus Munro)是我国西南热带亚热带地区重要的笋材两用竹种,具有很高的经济价值和重要的生态环境保护价值[1-2]。近期,作者利用云南甜龙竹和黄竹果实(种子)建立组织培养体系,并着手开展其种质遗传改良研究。常见的竹子果实大致可分为典型颖果、坚果状颖果、浆果状颖果三类[11],这2种竹子的种子均为坚果状颖果,表面光滑,且由于革质化果皮的存在,消毒液不易伤害种胚,因此,更适合进行表面消毒灭菌。利用竹子种子在无菌的条件下经严格消毒灭菌后培育成无菌苗,然后把无菌苗的茎尖、茎段、叶片等作为外植体材料,是近年来解决竹类植物外植体消毒灭菌难的有效方法之一[12]。为了解决云南甜龙竹、黄竹在组培过程中无菌操作难的问题,本研究以种子为实验材料,探讨其最佳表面灭菌条件和萌发特性,以期为组培体系的建立以及其种质遗传改良研究打下基础。
HTML
-
2018年3—4月,在云南红河州和西双版纳采集成熟云南甜龙竹、黄竹种子,种子采后自然晾干,剥去种子附着的稃片,4℃保存。选择颜色鲜亮,颗粒较大且饱满的完整种子备用。
-
将种子用流水冲洗6 h后置于超净工作台,经75%酒精浸泡10 s后并用无菌水冲洗6次,随后用不同消毒剂组合进行灭菌,每种消毒剂处理5 min,每次使用消毒剂后,都用无菌水冲洗6次,最后用无菌滤纸吸干表面水分,水平接种于MS培养基。每瓶接种2粒种子,每45瓶为1个重复,每处理设置3个重复(以下接种于MS培养基的均做此处理)。
实验处理分别为处理1:2%NaClO+0.01%HgCl2;处理2:3%NaClO+0.01%HgCl2;处理3:4%NaClO+0.01%HgCl2;处理4:2%NaClO+0.1%HgCl2;处理5:3%NaClO+0.1%HgCl2;处理6:4%NaClO+0.1%HgCl2。
-
选用流水冲洗时间(6、12、18 h)、2%NaClO浸泡时间(5、10、15 min)和0.1%HgCl2浸泡时间(5、10、15 min),采用L9(34)正交表设计三因素三水平实验(表 1)。将种子用流水冲洗后放于超净台→75%酒精浸泡10 s→无菌水冲洗6次→2%NaClO浸泡→无菌水冲洗6次→0.1%HgCl2浸泡→无菌水冲洗6次。在用消毒液浸泡的过程中,不断摇晃,以保证种子与消毒液充分接触。将消毒后的种子用无菌滤纸吸干表面水分,水平接种于MS培养基。
处理
Treatment因素 Factor 发芽率/%
Germination rate污染率/%
Pollution rate流水冲洗时间
Water flushing time2%NaClO浸泡时间
2%NaClO soaking time0.1%HgCl2浸泡时间
0.1%HgCl2 soaking time竹种 Bamboo species B M B M 1 1(6 h) 1(5 min) 1(5 min) 83.3±2.1 80.0±0.0 60.0±3.3 63.3±3.4 2 1 2(10 min) 2(10 min) 86.7±3.5 76.7±3.4 20.0±3.3 30.0±3.3 3 1 3(15 min) 3(15 min) 50.0±3.8 43.3±3.4 16.7±3.4 23.3±0.0 4 2(12 h) 1 2 86.7±3.9 76.7±3.4 43.3±3.4 50.0±3.3 5 2 2 3 83.3±3.4 66.7±3.4 20.0±3.3 26.7±0.0 6 2 3 1 43.3±0.0 50.0±3.3 33.3±0.0 40.0±3.3 7 3(18 h) 1 3 66.7±3.4 50.0±0.0 53.3±3.4 53.3±3.4 8 3 2 1 70.0±3.3 43.3±3.4 40.0±0.0 43.3±3.4 9 3 3 2 33.3±3.4 26.7±3.4 30.0±3.3 33.3±0.0 项目Project 竹种 Bamboo species B M B M B M 发芽率均值
Mean value of germination rate/%水平1 Level 1 73.3±17.4a 66.7±17.7a 78.9±10.2a 68.9±14.3a 65.5±18.1a 57.8±17.1a 水平2 Level 2 71.1±21.4a 64.5±12.0a 80.0±8.2a 62.2±15.1a 68.9±27.1a 60.0±25.2a 水平3 Level 3 56.7±17.8b 40.0±10.7b 42.2±8.1b 40.0±10.8b 66.7±14.3a 53.3±10.7a 污染率均值
Mean value of pollution rate/%水平1 Level 1 32.2±21.1A 38.9±18.7A 52.2±7.8A 55.5±6.7A 44.4±12.1A 48.9±11.3A 水平2 Level 2 32.2±10.4A 38.9±10.4A 26.7±10.3B 33.3±8.0B 31.1±10.5B 37.8±9.6B 水平3 Level 3 41.1±10.4A 43.3±9.0A 26.7±8.0B 32.2±7.5B 30.0±17.8B 34.4±14.3B 注:表中每列不同小写字母表示不同水平间发芽率差异显著(P<0.05),不同大写字母表示不同水平间污染率差异显著(P<0.05),下同。表中B代表云南甜龙竹,M代表黄竹,下同。
Notes: The difference between the small letters after each column data indicates germination rates of treatment levels are significant difference at 0.05 probability level. The difference between the capital letters after each column data indicates contamination rates of treatment levels are significant difference at 0.05 probability level. The same as follows. In the table, B represents D. brandisii, and M stands for D. membranaceus. The same as follows.Table 1. Results and analysis of orthogonal test on seed disinfection of D. brandisii and D. membranaceus
-
将种子洗净后用蒸馏水浸泡6 h,接种于垫有2层湿润滤纸的培养皿中,设3个重复,每个重复接种90粒种子。培养条件为8 h黑暗,16 h光照,温度(25±2)℃,相对湿度80%,实验过程中保持滤纸和种子湿润。
将种子洗净后分别用蒸馏水、3 mg·L-1 GA3浸泡6 h,然后用筛选出的最佳表面灭菌方式对其进行灭菌,分别接种于MS培养基。
-
当胚根长度≥2 mm时视为发芽,30 d内对种子的发芽率和污染率进行统计。
数据分析由SPSS17.0以及Excel统计软件完成。发芽率、污染率计算公式如下:
发芽率=发芽种子总数/试验种子总数×100%
污染率=污染种子总数/试验种子总数×100%
1.1. 试验材料
1.2. 试验方法
1.2.1. NaClO、HgCl2最佳消毒浓度组合
1.2.2. 云南甜龙竹、黄竹种子最佳表面灭菌时间
1.2.3. 云南甜龙竹、黄竹种子萌发特性
1.3. 数据统计与分析
-
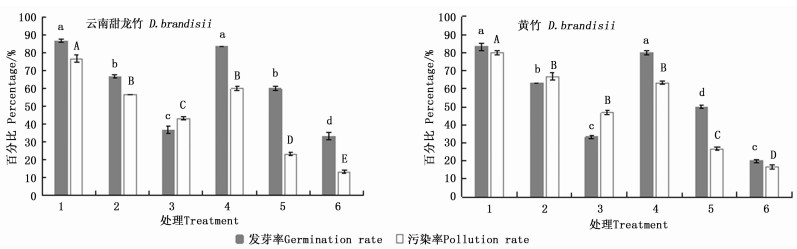
云南甜龙竹、黄竹种子均在2%NaClO+0.01%HgCl2(处理1)消毒处理后发芽率最高,分别为86.7%、83.3%,此时的污染率也最高,分别为76.7%、80.0%(图 1)。经4%NaClO+0.1%HgCl2处理(处理6),云南甜龙竹、黄竹种子的污染率最低,分别为13.3%、16.7%,但种子的发芽率很低,分别为33.3%、20.0%。方差分析的结果显示:在2个竹种中,多数处理之间的发芽率、污染率差异显著,处理1和4的发芽率均显著高于其它处理,但处理4的污染率显著低于处理1(图 1)。保持较高发芽率的同时应该尽量降低污染率[13],因此,处理4(2%NaClO+0.1%HgCl2)更适合这2种竹子种子的消毒,此时2种竹子种子的发芽率分别为83.3%、80.0%,污染率分别为60.0%、63.3%。
-
表 1表明:在9个处理中,云南甜龙竹处理2、4的发芽率最高,均为86.7%,污染率分别为20.0%、43.3%;处理3的污染率最低,为16.7%,此时的发芽率较低,仅为50.0%(表 1)。黄竹在9个处理中,发芽率最高为80.0%,此时的污染率也较高,为63.3%(处理1);处理3的污染率最低,为23.3%,但发芽率仅为43.3%。
除处理7云南甜龙竹与黄竹的污染率相同外(表 1),其他相同处理黄竹的污染率都比云南甜龙竹高,这可能是由于黄竹种子有一条纵沟槽,容易藏菌且不易彻底消毒灭菌,造成黄竹的污染率普遍较高。
种子不同消毒灭菌处理的发芽率方差分析的结果(表 2)表明:流水冲洗时间(18 h内)、2%NaClO浸泡时间(15 min内)对2种竹子种子的发芽率影响显著,即随着处理时间的延长,其发芽率显著降低,而0.1%HgCl2浸泡时间(15 min内)对其发芽率影响不显著。采用S-N-K法,对流水冲洗时间、2%NaClO浸泡时间进行各因素水平间的多重比较,结果(表 1)表明:流水冲洗时间的水平1(6 h)、2(12 h)均与水平3(18 h)差异显著,2%NaClO浸泡时间的水平1(5 min)、2(10 min)都与水平3(15 min)差异显著。
变异来源
Source of variation平方和 SS 自由度 Df 均方 MS F值 F Value 显著性 Significance B M B M B M B M B M 流水冲洗时间 Water flushing time 0.049 0.131 2 2 0.025 0.066 28.245 40.454 * * 2%NaClO浸泡时间 2%NaClO soaking time 0.278 0.137 2 2 0.139 0.069 159.717 42.282 * * 0.1%HgCl2浸泡时间 0.1%HgCl2 soaking time 0.002 0.007 2 2 0.001 0.003 1.013 2.144 - - 误差 Error 0.002 0.003 2 2 0.001 0.002 总变异 Total variation 4.374 3.208 9 9 注:“—”表示差异不显著(No significant difference);“*”表示0.05水平差异显著(Significant difference at 0.05 levels);下同。The same as follows. Table 2. Variance analysis of germination rate of D. brandisii and D. membranaceus by orthogonal test
种子消毒灭菌处理后污染率的方差分析结果(表 3)显示:2%NaClO浸泡时间(15 min内)、0.1%HgCl2浸泡时间(15 min内)对云南甜龙竹、黄竹种子的污染率影响显著,即随着处理时间的延长,其污染率显著降低,与正交试验结果一致;而流水冲洗时间(18 h内)对其污染率影响不显著。采用S-N-K法,对2%NaClO浸泡时间、0.1%HgCl2浸泡时间进行各因素水平间的多重比较,结果(表 1)表明:2%NaClO浸泡时间的水平1(5 min)与水平2(10 min)、3(15 min)差异显著,0.1%HgCl2浸泡时间的水平1(5 min)与水平2(10 min)、3(15 min)差异显著。
变异来源
Source of variation平方和 SS 自由度 Df 均方 MS F值 F Value 显著性 Significance B M B M B M B M B M 流水冲洗时间 Water flushing time 0.016 0.004 2 2 0.008 0.002 9.157 3.911 - - 2%NaClO浸泡时间2% NaClO soaking time 0.130 0.104 2 2 0.065 0.052 75.652 104.111 * * 0.1%HgCl2浸泡时间0.1% HgCl2 soaking time 0.039 0.034 2 2 0.019 0.017 22.472 34.348 * * 误差 Error 0.002 0.001 2 2 0.001 0.000 总变异 Total variation 1.300 1.609 9 9 Table 3. Variance analysis of pollution rate of D. brandisii and D. membranaceus by orthogonal test
发芽率和污染率是本研究着重考虑的2个方面,本试验结果显示:流水冲洗时间只对发芽率有显著性影响,随着时间的延长,发芽率显著降低,所以流水冲洗的最佳时间为6 h。2%NaClO浸泡时间对发芽率和污染率均影响显著,为保持较高发芽率的同时,尽量降低污染率[13],该处理的最佳时间为10 min。0.1%HgCl2浸泡时间只对污染率影响显著,随着时间的延长污染率显著降低,因此,0.1%HgCl2浸泡的最佳时间为15 min。在预实验中,种子只用0.1%HgCl2浸泡20 min后接种于MS培养基,1周后种子开始发黑,不能正常发芽,因此,本研究0.1%HgCl2浸泡时间最高设置为15 min。云南甜龙竹、黄竹种子表面灭菌的最佳方式为:种子经流水冲洗6 h置于超净台→75%酒精浸泡10 s→无菌水冲洗6次→2%NaClO浸泡10 min→无菌水冲洗6次→0.1%HgCl2浸泡15 min→无菌水冲洗6次。以此处理进行实验云南甜龙竹的发芽率为84.4%,污染率为18.7%,黄竹的发芽率为72.2%,污染率为29.6%。
-
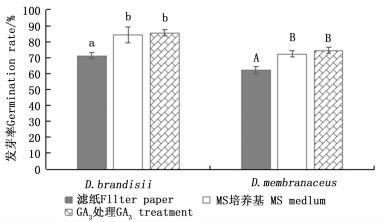
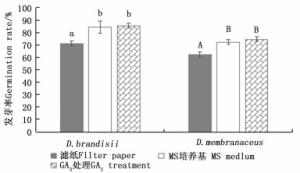
云南甜龙竹、黄竹种子在滤纸上的发芽率分别为71.1%和62.2%,在MS培养基上的发芽率分别为84.4%和72.2%;经3 mg·L-1 GA3处理后,在MS培养基上的发芽率分别为85.6%、74.4%(图 2)。t检验结果显示:在MS培养基上,未经GA3处理的云南甜龙竹种子与经3 mg·L-1 GA3处理的种子发芽率差异不显著,但这二者的发芽率均显著高于滤纸上的发芽率,黄竹种子也表现出了相同的规律。另外,云南甜龙竹和黄竹种子经过3 mg·L-1GA3处理后,在MS培养基上,第3 d开始伸出胚根,从伸出胚根到完成发芽仅需3~4 d;而在MS培养基上的种子第5 d开始伸出胚根,从伸出胚根到完成发芽需要7~8 d。在相同的处理下,云南甜龙竹发芽率比黄竹发芽率高10%左右,这可能是由竹种生理特性或种子质量差异造成的。
2.1. NaClO、HgCl2最佳消毒浓度组合
2.2. 云南甜龙竹、黄竹种子最佳表面灭菌时间
2.3. 云南甜龙竹、黄竹种子萌发特性
-
由于外植体的种类不同,沾染的病菌种类和严重程度差异较大,选择消毒剂的种类也不同,常用的消毒剂有KMnO4、0.01%~0.2%HgCl2、0.25%~4%NaClO,70%~75%酒精、有效氯、H2O2等[10, 12]。消毒剂的浓度对外植体的彻底消毒至关重要,浓度过高会对外植体造成伤害,浓度太低达不到理想的消毒效果。毛竹(Phyllostachys heterocycla (Carr.) Mitford cv. pubescens)、云南龙竹(Dendrocalamus yunnanicus Hsuch et D. Z. Li)种子表面灭菌最适合的消毒剂种类和浓度分别为2.5%NaClO[14]和75%酒精+0.01%HgCl2[15];而2%NaClO+0.1%HgCl2最适合花叶矢竹(Pseudosasa japonica cv. akebono-suji)侧芽茎尖的消毒[16]。因此,根据竹种和外植体类型选择恰当的消毒剂种类和浓度十分重要。本研究筛选出云南甜龙竹和黄竹种子表面杀菌的最佳消毒浓度组合为2%NaClO+0.1%HgCl2,此时云南甜龙竹和黄竹的发芽率分别为83.3%、80.0%,污染率分别为60.0%、63.3%。
-
合适的消毒时间也是外植体表面灭菌的关键[17],不同消毒剂适宜的处理时间也不一样,理想的状况是在保持较高发芽率的同时,尽量降低污染率[13]。联合使用0.2%NaClO浸泡15 min、0.1%KMnO4浸泡12 h、0.1%HgCl2浸泡30 min等处理对慈竹(Neosinocalamus affinis (Rendle)Keng f)种子灭菌,污染率可控制在6%左右[12];云南龙竹种子[15]经75%酒精处理15 s和0.01%HgCl2处理3 h后,污染率可降低到16.7%;而单独使用0.25%NaClO、0.1%HgCl2分别对毛竹、孝顺竹种胚灭菌5 min和20~30 min,也可达到较好的消毒效果[18-19]。本研究筛选出2种竹种最佳表面灭菌时间组合均为:流水冲洗6 h、2%NaClO浸泡10 min、0.1%HgCl2浸泡15 min,此时云南甜龙竹、黄竹的发芽率分别为84.4%、72.2%,污染率分别控制在18.7%、29.6%。
种子用流水冲洗可冲走其表面的灰尘和其他粘附物,同时也能让其吸足水分,提高种子的发芽率[20]。本研究结果显示,流水冲洗时间对种子的发芽率影响显著,当冲洗时间超过12 h,种子的发芽率显著降低,污染率升高。这可能是由于吸水膨胀过度的种子,在随后的次氯酸钠溶液处理中,容易灼伤种胚[7];同时种皮破损,微生物可能侵入,进而影响灭菌效果[18]。2%NaClO浸泡时间对种子的发芽率和污染率均影响显著,当浸泡时间超过10 min时,种子的发芽率显著下降,这可能是长时间的NaClO处理会对种子产生了较强的药物伤害。
-
云南甜龙竹、黄竹的种子在MS培养基与经3 mg·L-1 GA3处理后在MS培养基上的发芽率都显著高于滤纸上的发芽率。滤纸发芽实验中,种子容易发霉而被真菌菌丝包围,其中,部分真菌会感染胚根,使其失去活性,使得滤纸上的种子发芽率降低,这与毛竹种子的情况相似[13, 21]。云南甜龙竹、黄竹种子在MS培养基上的发芽率分别为为84.4%、72.2%,且2种竹子种子在MS培养基上第5天开始伸出胚根,约13 d完成发芽,显示其种子不存在休眠期[22-23]。GA能诱导禾本科种子糊粉层细胞中α-淀粉酶、蛋白酶等水解酶的大量释放,水解胚乳中的贮藏物质,为种子的萌发提供能量和物质,促进种子的发芽[24]。本研究中,经3 mg·L-1GA3处理后的竹子种子,伸出胚根的时间缩短2 d,且完成发芽的时间较短,显示GA3对2种竹子种子发芽具有促进作用。2种竹种各自在MS培养基上的发芽率与经3 mg·L-1GA3处理后在MS培养基上的发芽率差异不显著,这可能是由于它们的种子不存在休眠期,而且新鲜采集的种子本身具有较高的活力[22]。
3.1. NaClO、HgCl2最佳消毒浓度组合
3.2. 云南甜龙竹、黄竹种子最佳表面灭菌时间
3.3. 云南甜龙竹、黄竹种子萌发特性
-
云南甜龙竹和黄竹种子NaClO、HgCl2最佳消毒浓度组合为:2%NaClO+0.1%HgCl2。云南甜龙竹和黄竹种子表面灭菌的最佳方式均为:种子用流水冲洗6 h放于超净台→75%酒精浸泡10 s→无菌水冲洗6次→2%NaClO浸泡10 min→无菌水冲洗6次→0.1%HgCl2浸泡15 min→无菌水冲洗6次,此时,2种竹子种子的污染率可分别控制在18.7%、29.6%。云南甜龙竹和黄竹种子在MS培养基上的发芽率分别为84.4%和72.2%;GA3处理可以促进2种竹子种子的发芽。本研究为云南甜龙竹、黄竹组培体系的建立奠定了基础。






 DownLoad:
DownLoad: