-
油茶(Camellia oleifera Abel.)属山茶科(Theaceae)山茶属(Cameuia)植物,是我国主要的经济林树种,也是世界四大木本油料植物之一[1]。由于油茶具有适应性强、抗逆性好的特性,十分适宜在相对贫瘠的红壤丘陵地区种植,因此广泛分布于我国湖南、江西、广西等17个省区,在我国已有超过两千年的种植历史。我国现有油茶林种植面超过4.3×106 hm2,油茶籽年产达217万吨,产值超过600亿元。湖南作为油茶的最大产区和茶油的最大消费区,油茶资源约占全国40%[2]。但是,油茶的种植生产过程中往往受到多种病虫害的侵袭,导致油茶的产量和品质大幅下降,其中以油茶炭疽病对油茶林的危害最为严重。
油茶炭疽病(Colletotrichum camellia Massee)病菌能够侵染油茶的花芽、叶芽、果实、枝梢和叶片,通常引起油茶的落花、落果和枝枯现象,严重时甚至能造成整株死亡;在我国各油茶产区均有发生,可导致20%左右的平均落果率,有时可高达40%~50%,是油茶种植过程中的最主要病害之一[3]。尽管化学农药对油茶炭疽病的防治具有一定效果[4-7],但长期滥用化学农药会引起病原菌的抗药性,造成环境污染,同时大幅增加成本[8-9]。因此,开发出对油茶炭疽病防治效果显著,无污染、无公害、可持续的生物防治手段显得尤为重要。
芽孢杆菌属中的解淀粉芽孢杆菌(Bacillus amyloliquefaciens)在自然界中分布广泛,对人、畜无毒无害,具有很高的抗逆能力;研究表明,解淀粉芽孢杆菌具有广谱的抑制真菌和细菌活性的作用,且生长速度快、稳定性强,是一种理想的生防菌资源[10-11]。解淀粉芽孢杆菌的主要生防机制包括有定殖、分泌拮抗物质、诱导系统抗性等,其中分泌拮抗物质如脂肽类抗生素和挥发性物质等,可显著抑制病原微生物的活性,抑制病害的发生[12-13]。因此,本研究分别通过高效液相色谱法(HPLC)和气相色谱-质谱法(GC-MS)对前期研究工作中分离得到的解淀粉芽孢杆菌菌株P-14中的脂肽类抗生素和挥发性物质分别进行分离、鉴定,以期获得高效的抑菌物质用于油茶炭疽病的生物防治。
HTML
-
解淀粉芽孢杆菌菌株P-14由湖南省林业科学院森林资源保护所分离鉴定,油茶炭疽病病原真菌(C. Camellia)由湖南省林业科学院森林资源保护所分离保存。
-
将保存的解淀粉芽孢杆菌菌株P-14划线到LB培养基(Luria-Bertani Broth)平板上,37℃下活化24 h;而后挑取活化菌株的单菌落接种于含100 mL LB液体培养基的三角瓶中,37℃、170 r·min-1下培养24 h作为种子液。将5 mL种子液接种到100 mL Landy培养基中,30℃、170 r·min-1下震荡培养,连续培养60 h后获得发酵液,将发酵液置于低温条件下12 000 r·min-1离心初步除去菌体,并用0.22 μm无菌微孔滤膜过滤。取50 μL滤液均匀涂布于LB培养基平板上,37℃下培养24 h无杂菌即得无菌滤液。
-
通过酸沉淀法[14]提取无菌滤液中的脂肽类抑菌物质,加入6 mol·L-1 HCl调节无菌滤液pH至2.0,4℃过夜沉淀;8 000 r·min-1下离心10 min,弃上清液,待沉淀自然干燥后加入甲醇抽提两次并将抽提液合并,即得脂肽抗菌物质粗提物;粗提物冷冻干燥后溶于pH 6.8的磷酸缓冲液(PBS)中,用0.22 μm的无菌滤膜过滤,置于-20℃下备用。
-
脂肽类抑菌物质的纯化分离采用高效液相色谱法,分离柱为Eclipse XDB-C18(4.6×250 mm,Agilent,USA);流动相A为0.1%的乙酸溶液,B为100%乙腈,A:B=60:40;流速设置为0.4 mL·min-1,柱温为30℃,进样量为20 μL。
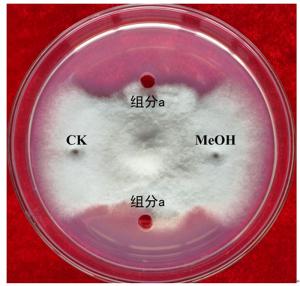
采用抑菌圈法筛选具有抑菌效果的HPLC分离物,用已灭菌的打孔器在PDA平板上等距地打四个小孔,分别取100 μL HPLC分离物加入小孔中,对照组为无菌水和甲醇溶液;每个分离物重复试验3次,培养7 d后观察平板上的抑菌圈大小以确定该组分是否具有抑菌作用。
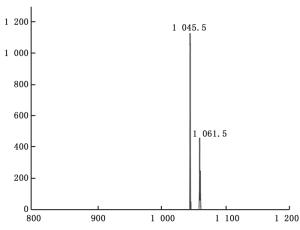
通过抗菌效果实验筛选出的拮抗物质,运用安捷伦高效液相色谱-电喷雾/质谱仪(HPLC-ESI-MS;HPLC:1200系列,ESI-MS:6410 Triple Quad LC/MS,USA)进一步鉴定其分子量与结构。分离柱为MS C18色谱柱(50 mm×2.1 mm,1.8 μm,Agilent,USA)。溶剂A为乙腈,B为水(含0.1%乙酸),A:B =40%:60%。流速设置为0.3 mL·min-1,柱温为25℃,210 nm波长检测。电离方式采用电喷雾离子源,质量检测器为四级质量分析器。电喷雾条件为:喷雾电压4.5 kV、毛细管温度300℃,检测方式为正离子模式(ESI+),利用碰撞诱导解离技术(CID)获得化合物的典型碎片离子,碰撞池中碰撞气体为氮气,碰撞能量根据试验需要进行设定。
-
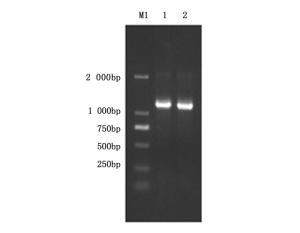
采用CTAB法提取菌株P-14的基因组DNA,以此为模板扩增脂肽类抗生素bacillomycin D合成的相关基因bamD,其引物(F: 5’-GCTCTTGATGACCTTCTCTGCG-3’,R: 5’-ATTTTGTGCGATTGGTTCTGTT-3’)选择参考Chen et al.[15]中所述。将扩增的基因片段测序后在NCBI上进行BLAST同源性分析,判定拮抗菌P-14是否能够合成期望的拮抗物质。
-
在二分格培养皿的两侧分别倾倒LB固体培养基和PDA固体培养基,划线接种P-14菌株到二分格培养皿一侧的LB培养基中央,另一侧同时接种的油茶炭疽病的病原菌菌饼于PDA培养基中央,重复试验3次,对照组中不接种P-14菌株。置于25℃下培养3 d,逐日记录每天病原菌的菌落直径。
-
在二分格培养皿的一侧倾倒LB固体培养基,划线接种P-14菌株,另一侧放置灭菌滤纸,滴入适量无菌水保湿;对照皿中不接种P-14菌株。取油茶健康叶片用无菌水冲洗晾干后置于灭菌滤纸一侧,用无菌针头在叶片中央划出一条伤口,并将油茶炭疽病的病原菌菌块接种于伤口处,重复试验3次,在28℃下培养3 d,观察油茶叶片的发病情况。
-
采用顶空固相微萃取法(head-space solid-phase micro-extraction,HS-SPME)收集P-14菌株产生的挥发性物质。挑取P-14菌株的菌块接种于含200 mL PDA培养基的锥形瓶中,而后将50/30 μm DVB/PDMS萃取头(Supelco,Vienna,Austria)置于锥形瓶的顶部空间,并暴露于P-14菌株产生的挥发性物质(VOCs,volatile organic compounds)中,以不接种P-14菌块的PDA液体培养基作为空白对照。将锥形瓶置于30℃下培养7 d,而后取出含VOCs的萃取头,注入气相色谱-质谱联用仪(GC-MS,Gas Chromatography-Mass Spectrometry)中进行检测。将含VOCs的萃取头在220℃下加热1 min释放VOCs,而后以He作为载气,恒速1 mL·s-1进行监测,首先将萃取柱在220℃加热1 min将VOCs释放于HP-5MS色谱柱(Agilent,Waldbronn,Germany)中,然后再用He作为载气恒速1 mL·min-1进行检测。检测程序为初始温度40℃,保持2 min,而后以10℃·min-1的速率升温到200℃;接着以25℃·min-1的速率升温到260℃,保持5 min。质谱检测参数参考Yuan et al.[16],将测定的各物质质谱信息与Mainlib和Replib质谱数据库条目进行比较,如果匹配度达≥90%即可认定为该种物质。
1.1. 供试菌株
1.2. 菌液制备
1.3. 脂肽类抗生素的测定
1.3.1. 脂肽类抑菌物质的提取
1.3.2. 脂肽类抑菌物质的HPLC分离及鉴定
1.3.3. 拮抗物质合成的相关基因检测
1.4. 挥发性有机物质(VOCs)的测定
1.4.1. 挥发性抑菌物质的平板检测
1.4.2. 挥发性抑菌物质的离体叶片抑菌试验
1.4.3. 挥发性抑菌物质的收集及检测
-
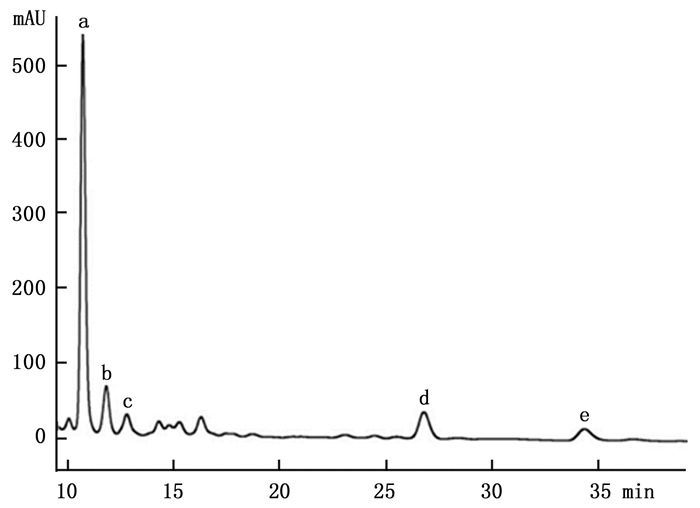
通过HPLC分析,从解淀粉芽孢杆菌P-14发酵液中纯化收集到5种主要组分,其分析图谱如图 1所示;对收集的5种组分进行冷冻干燥后,溶于一定量的甲醇中备用。运用牛津杯法分别测定5种组分的抗菌活性,抗菌效果如图 2所示,结果表明5种主要成分中仅有a号峰有抑菌活性。将收集的a号峰进行HPLC-ESI-MS分析,得到分子量分别为1 045.5和1 067.5的物质,其质谱图如图 3所示。对比芽孢杆菌脂肽物质的分子量数据可知:组分a中主要是含有C15 bacillomycin D(杆菌霉素D)。
-
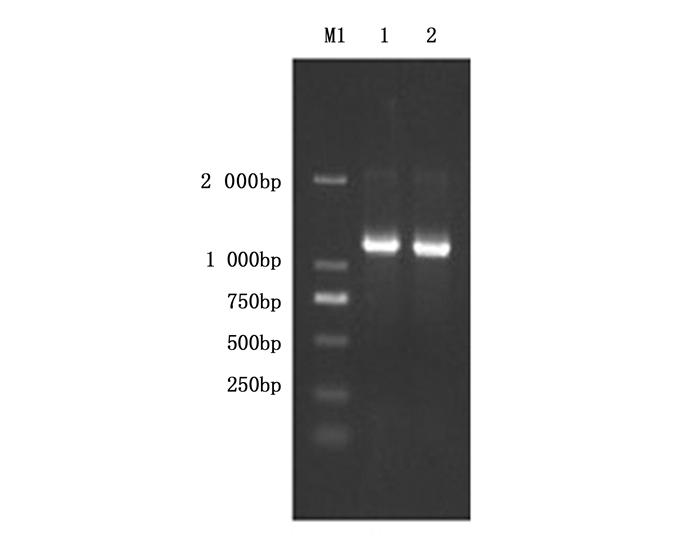
为进一步确定拮抗菌P-14产生的脂肽类拮抗物质,以菌株P-14的基因组DNA为模板扩增bacillomycin D合成的相关基因,其PCR产物的大小和理论值吻合,结果如图 4。将扩增得到的序列上传至NCBI进行同源性分析,比对结果表明菌株P-14中bamD基因片段与B. amyloliquefaciens FZB42 bacillomycin D基因同源性达99%。
-
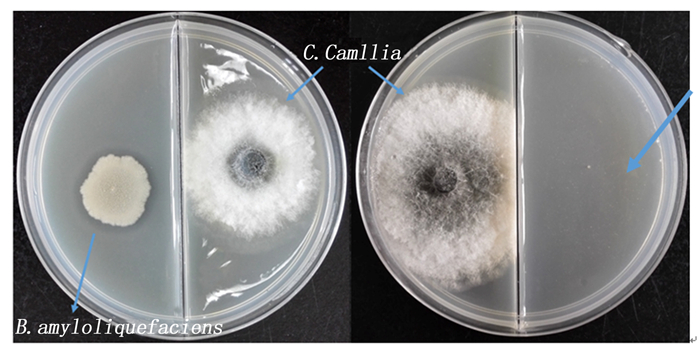
挥发性物质抑菌试验结果如图 5所示,接种3 d后,解淀粉芽孢杆菌处理中病原菌的菌落直径显著低于对照组,相对抑菌率为(26.19±3.82)%;此外,经过3次重复试验,在油茶健康叶片发病实验中,二分皿另一侧未接种P-14菌株时,油茶叶片发病严重,而接种P-14菌株的油茶叶片未发病(图 6),表明解淀粉芽孢杆菌的挥发性物质具有显著的抑菌活性。运用顶空固相微萃取法收集P-14菌株产生的挥发性气体进行GC-MS分析,可分离得到35种化合物,包含苯类物质(benzenes)10种、烷基类物质(alkyls)8种、醇类物质(alcohols)2种、酮类物质(ketones)11种、醛类物质(aldehydes)3种和1种酯类物质(esters),如表 1所示。

Figure 5. B. amyloliquefaciens anti-fungal volatile activity on divided plates. Mycelial plug growth was inhibited in the presence of the bacteria streaked in the different compartments (right), compared to the control (left)

Figure 6. Volatile organic compounds of B. amyloliquefaciens reduce symptoms of Camellia oleifera leaf. Mycelial plug growth was inhibited in the presence of the bacteria streaked in the different compartments (right), compared to the control (left)
苯类物质Benzenes 烷基类物质Alkyls 醇类物质Alcohols 酮类物质Ketones 醛类物质Aldehydes 酯类物质Esters 保留时间
Retention time化合物
Compounds保留时间
Retention time化合物
Compounds保留时间
Retention time化合物
Compounds保留时间
Retention time化合物
Compounds保留时间
Retention time化合物
Compounds保留时间
Retention time化合物
Compounds1.25 1, 4-Pentadiene 12.64 Cyclooctane, methyl- 10.48 Benzyl Alcohol 7.68 2-Heptanone 9.16 Benzaldehyde 14.51 n-pentadecyl ester 9.54 Phenol 13.05 Dodecane 10.35 1-Hexanol, 2-ethyl- 8.96 2-Heptanone, 6-methyl- 10.64 Benzaldehyde, 2-hydroxy- 10.09 Benzene, 1, 2-dichloro- 15.48 Cyclodecane 10.79 2-Nonanone 15.97 Dodecanal 11.21 Pyrazine, 2-ethyl-3, 5-dimethyl- 15.83 Tetradecane 11.04 Acetophenone 13.57 Benzothiazole 16.68 2-Methyl-1-dodecene 11.39 2-Nonanone 14.75 Pyrazine, 2, 5-dimethyl-3-(3-methylbutyl)- 16.79 Cyclododecane 12.40 2, 4, 6-Cycloheptatrien-1-one 15.24 Quinazoline, 4-methyl- 19.52 Eicosane 13.47 Benzofuran, 2-ethenyl- 16.25 Naphthalene, 2, 6-dimethyl- 20.06 Nonadecane 13.90 2-Undecanone 17.00 9-Octadecene, (E)- 15.3 4-Dodecanone 17.35 Butylated Hydroxytoluene 16.60 2-Tridecanone 17.84 2-Hexadecanone Table 1. Volatile organic compounds produced by B. amyloliquefaciens P-14
2.1. 拮抗菌P-14脂肽类物质的分离与鉴定
2.2. 拮抗菌P-14与脂肽类拮抗物质合成相关基因的检测
2.3. 解淀粉芽孢杆菌挥发性抑菌物质的鉴定
-
解淀粉芽孢杆菌在促进植物生长、增强植物抗病性方面具有一定作用,并作为拮抗菌在棉花、番茄、黄瓜等的病虫害防治中得到广泛应用[17-19]。解淀粉芽孢杆菌能产生多种具有拮抗作用的次生代谢产物,主要可分为两大类:通过核糖体途径合成的抗菌物质,如细菌素[20-21]及杆菌溶素[22]等;通过非核糖体途径也能合成两种重要抗菌物质,如脂肽类抗生素[23]、聚酮化合物[24]和挥发性抗菌物质[16]。本研究对解淀粉芽孢杆菌菌株P-14主要抗菌活性物质——脂肽类抗生素和挥发性物质进行了分离纯化,并进行鉴定,以期阐明该菌株的生防机制。
研究表明,解淀粉芽孢杆菌可以产生至少一种具有拮抗作用的脂肽类抗生素,其中两个主要类群——伊枯草杆菌素Iturins和丰原素Fengycins具有很强的抗真菌活性[25-27]。本研究利用酸沉淀法和HPLC法分离鉴定解淀粉芽孢杆菌P-14发酵液中的脂肽类物质,结果表明分离后的组分a对油茶炭疽病的病原菌具有良好的抑制作用,组分a中主要成分为分子量1 045.5和1 067.5的C15 bacillomycin D,该组分属于伊枯草菌素家族Iturin,是一种在抑制真菌生长方面起主要作用的胞外物质[28]。研究表明,bacillomycin D是抑制黄曲霉(Aspergillus flavus)效果最佳的抗生素之一[29],对白粉病病原菌(Podosphaera fusca)也具有很强的抑制作用[30];与其它脂肽类抗生素无交叉耐药性、毒性小、抗菌谱广、显著增强其他脂肽类抗生素的抑菌活性[31]。信珊珊等[32-33]研究表明,运用解淀粉芽孢杆菌WH1中的脂肽类物质作为防治油茶炭疽病的生物农药是可行的,但未对其主要有效成分进行分离鉴定;孟庆敏等[34]通过乙醇沉淀、固相萃取、HPLC及LC-MS对枯草芽孢杆菌Y13中的抑菌物质进行分离鉴定,结果表明其主要脂肽类抑菌物质分别为Iturin和Fengycin的同系物。本研究首次从油茶分离并鉴定解淀粉芽孢杆菌中脂肽类抑菌物质bacillomycin D,并验证了其对油茶炭疽病的抑制效果。
芽孢杆菌所合成的挥发性物质的抑菌作用明显,代谢产生的挥发性物质能够抑制多种植物病原真菌的生长,其中包括酸、酮、酯和醇等有机物,不同的挥发性物质抑制真菌的效果也不同[35-37]。本研究中通过顶空固相微萃取法收集,能够直接收集挥发性物质,无需采用有机溶剂萃取,既增加了收集的挥发性物质种类,同时也减少了操作步骤,提高了收集效率[27]。此外,本研究通过GC-MS对解淀粉芽孢杆菌P-14的挥发性物质进行了鉴定,共分离得到35种化合物,包含苯类物质(benzenes)10种、烷基类物质(alkyls)8种、醇类物质(alcohols)2种、酮类物质(ketones)11种、醛类物质(aldehydes)3种和1种酯类物质(esters),其种类与前人研究基本一致。本研究分离的35种化合物中,至少有13种在其他芽孢杆菌的挥发物中能够分离得到,其中苯并噻唑(Benzothiazole)、2-乙基己醇(1-Hexanol, 2-ethyl-)、2-壬酮(2-Nonanone)、苯酚(Phenol)、2-十一酮(2-Undecanone)已被证实具有植物生长的功能,并能诱导植物产生抗病作用[16, 38-39]。其它挥发性化合物是否具有抑菌作用,还需进一步的证实。
-
本研究通过对解淀粉芽孢杆菌菌株P-14产生的脂肽类抗生素的分离与鉴定,获得了对油茶炭疽病病原菌具有拮抗作用的组分a,其主要成分为C15 bacillomycin D;同时对P-14产生的挥发性物质进行分离验证,结果表明P-14产生的挥发性物质对油茶炭疽病病原菌具有拮抗作用,并分离得到了35种挥发性物质。本研究确定了解淀粉芽孢杆菌P-14的拮抗物质种类,为油茶炭疽病的生物农药开发提供了可靠的研究内容。





 DownLoad:
DownLoad: