-
植物在受到昆虫侵袭时会通过调节体内化学物质的组成和数量来降低其营养水平,从而对昆虫的取食产生拮抗[1],这些化学物质包括蛋白质、糖、氮、叶绿素等生理物质[2-4]。营养物质和次生代谢物质是植物化学防御系统的重要屏障,已经在多种寄主与害虫间被证实,如南美斑潜蝇[5](Liriomyza huidobrensis Blanchard)、松墨天牛[6](Monochamus alternatus Hope)、绿盲蝽[7](Apolygus lucorum Meyer-Dür)等昆虫在危害其寄主时,寄主叶片中的可溶性糖、蛋白质含量等物质均发生了变化,并且变化与危害程度也有一定的关系。通常植物在遭受植食性昆虫为害后,能通过自身的生理、生化及形态特征等多方面的变化而产生诱导抗虫性[8],如烟粉虱(Bemisia tabaci(Gennadius))取食可引起辣椒(Capsicum annuum L.)的营养物质和抗性物质含量向着有利于提高抗虫性的方向改变[9]。在害虫攻击寄主植物的过程中,昆虫产卵可作为早期受损信号诱导植株产生直接或间接抗性[10]。有研究表明, 在豌豆(Pisum sativum L.)豆荚中,豌豆象鼻虫(Bruchus pisorum L.)产卵会触发未分化细胞的生长,导致卵从叶片表面抬升,增加了卵干燥、被捕食或从豆荚上脱落的风险[11],这些产卵诱导的反应目的是去掉或杀死植物上的卵来避免卵孵化出幼虫造成的伤害[12-13],这都属于直接抗性,由卵块沉积物诱导的进一步直接抗性是已经产卵和邻近产卵的叶片对产卵雌虫形成威慑[14]。产卵作为激发子有能力诱导植物挥发物,已经证实带有卵块的豆科植物、榆树叶片和松针的萜类挥发物在数量和质量上都有变化[15-17]。比起被严重侵染的叶片,榆黄萤叶甲(Xanthogaleruca luteola Müller)雌虫更喜欢在未侵染的叶片上产卵[18];再者,被卵块沉积物诱导产生的间接抗性包括挥发物的释放或产卵叶片表面化学物质的改变从而吸引卵寄生蜂,如欧洲赤松(Pinus sylvestris L.)松枝在叶蜂(Diprion pini (L.))产卵后能释放挥发物吸引卵寄生蜂(Chrysonotomyia ruforum(Krausse))[19]。相似的三级营养关系在榆树(Ulmus spp.)[20]和菜豆(Phaseolus vulgaris Linn.)[21]上都已发现。另外,已有实验证实与产卵叶片邻近的未产卵叶片确实表现出了系统性的诱导抗性[20-22]。
杨小舟蛾(Micromelalopha sieversi (Staudinger))属于鳞翅目(Lepidoptera)舟蛾科(Notodontidae),该虫产卵量较大,以幼虫群集啃食叶片为主,危害严重时常在短期内将一片杨树林叶子吃光,是危害杨树最严重、治理最困难的害虫之一。近年来,围绕该虫开展了相关研究,包括羽化节律及其影响因子等方面[23]。昆虫产卵可以作为寄主植物产生抗性的一个信号,但是关于产卵后植株内含物的含量如何变化少有报道,并且产卵是否会诱导邻近植株产生抗性也未见报道。基于此,笔者选取杨树与杨小舟蛾为研究对象,其中,杨树以欧美杨108号(108杨)(Populus × euramericana ‘Guariento’)和欧美杨111号(111杨)(P. × euramericana‘Bellotto’)作为模式寄主,前人研究发现杨小舟蛾对108杨和111杨具有不同的产卵选择性[24]。通过杨小舟蛾的产卵处理,观察产卵植株和邻近植株对幼虫生长的影响,并测定各处理叶片内含物的含量变化,分析产卵植株以及与产卵植株邻近的植株是否产生诱导抗虫性,从寄主生理层面上揭示杨小舟蛾产卵能引起杨树产生抗性,为今后进一步探讨产卵行为与寄主抗性的关系奠定基础,并为该虫的综合治理提供新的视角和思路。
HTML
-
实验选用杨树品种为黑杨派无性系欧美杨108号(108杨)和欧美杨111号(111杨),杨树枝条采自北京市大兴区林业站苗圃(10年生108杨、107杨和111杨混交林),3月份将当年生扦插苗种植在装有营养土的花盆中,在室外进行栽培,定期浇水、除草、人工除虫(未喷施农药),8月份将生长基本一致的植株搬入实验室进行杨小舟蛾产卵实验。
-
实验所需杨小舟蛾蛹于2017年8月采自山东省平阴县10年生108杨树林。虫蛹采回后将雌、雄蛹单头分开放置于培养箱(温度(28±1)℃,湿度(50±1)%,光照L:D=16:8)待羽化。
1.1. 植物材料
1.2. 供试虫源
-
在一个实验室内搭建0.75 m ×0.75 m ×1.5 m (长×宽×高)的养虫笼2个,每个养虫笼放入4株108杨(盆栽扦插苗,树高、叶量基本一致的样本)分别作为产卵植株和邻近植株,植株相距约50 cm。同时在另一实验室内搭建同样大小的养虫笼,放入4株对照植株。2个实验室条件都是自然光周期、温度(26 ± 2)℃、湿度(50 ± 5)%。111杨采用同样的设置方法。
-
将20对刚羽化的杨小舟蛾雌雄虫放入待产卵植株的养虫笼内,自由交配产卵,在卵期(72 h)即将结束后采集带有卵块的叶片,同时采集邻近植株和对照植株对应部位的叶片。将叶片烘干过筛(60目)待用,用于测定叶片可溶性糖、全N、游离氨基酸、总酚和单宁含量,共设3个重复。
-
产卵植株自然孵化出的幼虫继续取食(50头),另外对邻近和对照植株接同龄幼虫(50头)喂养,在叶片接近吃光时统计幼虫存活数量。108杨和111杨的接虫方法相同。
-
参照郝学宁[25]的方法,准确称取样品0.20 g于25 mL容量瓶中,加蒸馏水25 mL,超声波震荡2 h,冷却后离心(10 000 r·min-1,5 min)取上清液待上机。仪器为Waters 2695液相色谱系统,色谱柱为sugar-pak 1,流动相为水(流速0.6 mL·min-1),检测器为示差检测器,柱温70℃。
-
参照章平泉等[26]的方法,准确称取样品0.20 g于消解管,先加入2粒混合催化剂片(硫酸钾:硫酸铜=10:1),再加入15 mL H2SO4,静置过夜,第2天在DK20凯氏消化炉中消解,冷却后使用UK152全自动凯氏定氮仪测定全N含量。
-
参照何轶等[27]的方法,准确称取样品0.20 g于25 mL容量瓶中,加蒸馏水25 mL,超声波震荡2 h,冷却后离心(10 000 r·min-1,5 min)取上清液待上机。取上清液10 μL, 用AccQ·Fluor试剂衍生。仪器为Waters 2695液相色谱系统,色谱柱为AccQ·Tag aa分析柱(3.9 mm×150 mm),流动相A为140 mmol·L-1 NaAc溶液(17 mmol·L-1三乙胺,pH=4.95),流动相B为60%乙晴水溶液(60:40),梯度洗脱,流速1 mL·min-1;检测器:荧光,Ex250, Em395 gain 10。
-
Folin-Donis比色法(FD法)[28]。准确称取干燥粉碎后的杨树叶片0.2 g放入50 mL三角瓶中,用10 mL70%乙醇在90℃水浴中反复提取4次,每次冷却后离心取上清液,最后滤液合并后用70%乙醇定容,待用。样品滤液经处理后于680 nm测OD值,同时用单宁酸制作标准曲线。
-
Folin-Cioealteu比色法(FC法)[29]。将叶片在液氮中磨碎,称取叶片样品1.0 g于150 mL三角瓶中,加入70%乙醇100 mL,超声波震荡1 h,取下静置过滤上清液,并用70 %乙醇定容到100 mL容量瓶中待测。样品粗提液经处理后于760 nm下测OD值,同时用没食子酸制作标准曲线。
-
采用Spss 19.0统计软件进行单因素方差分析(One-Way ANONA)和多重比较(LSD),Duncan方法进行样本间差异显著性分析,利用Excel 2007作图。
2.1. 不同处理植株的设置
2.2. 产卵处理
2.3. 幼虫取食产卵植株、邻近植株和对照植株的存活率
2.4. 测定方法
2.4.1. 可溶性糖含量的测定
2.4.2. 全N含量测定
2.4.3. 游离氨基酸含量测定
2.4.4. 单宁含量测定
2.4.5. 总酚含量测定
2.5. 统计方法
-
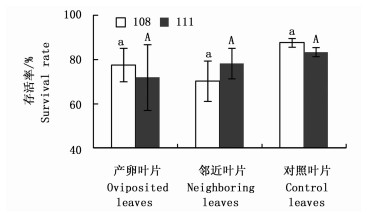
图 1表明:杨小舟蛾幼虫分别取食108杨和111杨产卵植株、邻近植株和对照植株的叶片,幼虫存活率均差异不显著。
-
108杨产卵植株和邻近植株的可溶性总糖的含量均显著低于对照植株;但产卵和邻近植株的多糖、葡萄糖、果糖和二糖的含量与对照植株均差异不显著;三糖只在产卵植株和邻近植株中检测到(表 1)。
mg·g-1 不同处理
Differenttreatments多糖
Polysaccharide三糖
Trisaccharide二糖
Disaccharide葡萄糖
Glucose果糖
Fructose总糖
Total sugar产卵植株Oviposited plants 150.40±21.27 a 0.65±0.65 a 18.49±6.97 a 10.32±4.94 a 18.34±5.92a 198.19±4.33 b 邻近植株Neighboring plants 160.49±7.97 a 0.75±0.75 a 17.78±5.29 a 5.75±1.47 a 16.48±1.91 a 201.25±2.66 b 对照植株Control plants 167.70±0.75 a 0 a 16.70±0.16 a 10.87±0.07 a 19.89±0.16 a 215.15±1.03 a 注:同列数据后不同小写字母表示差异显著(p < 0.05)。下同。
Note: Values with different small letter within the same line have significant difference(p < 0.05). The same below.Table 1. The content of soluble sugar in differenttreatments of '108'
111杨产卵植株的可溶性总糖含量显著低于邻近植株,但产卵和邻近植株与对照植株均差异不显著;产卵和邻近植株的二糖含量均显著低于对照植株,果糖和葡萄糖含量均与对照差异不显著;邻近植株的多糖含量显著高于产卵植株,但与对照差异不显著;111杨的3种处理中都未检测到三糖(表 2)。
mg·g-1 不同处理
Differenttreatments多糖
Polysaccharide三糖
Trisaccharide二糖
Disaccharide葡萄糖
Glucose果糖
Fructose总糖
Total sugar产卵植株Oviposited plants 162.15±15.67 b 0 8.21±1.48 c 11.86±3.25 a 15.28±1.65 a 197.51±13.27 b 邻近植株Neighboring plants 206.21±3.95 a 0 15.29±1.69 b 7.96±1.23 a 14.88±0.59 a 244.33±4.02 a 对照植株Control plants 167.70±10.61 ab 0 32.31±1.67 a 7.14±0.31 a 18.02±1.00 a 225.17±11.47 ab Table 2. The content of soluble sugar in differenttreatments of '111'
-
108杨的产卵植株和邻近植株的全N含量和总糖/全N均与对照植株差异不显著。邻近植株的游离氨基酸总量显著高于产卵和对照植株,产卵植株与对照差异不显著(表 3)。
不同处理
Differenttreatments全N
Total nitrogen
/%总糖/全N
Total sugar/
total nitrogen游离氨基酸总量
Total free amino
acids/(mg·g-1)产卵植株
Oviposited plants2.23±0.51 a 9.29±2.64 a 2.60±0.33 b 邻近植株
Neighboring plants2.80±0.70 a 7.53±2.10 a 7.44±0.17 a 对照植株
Control plants3.07±0.23 a 7.04±0.60 a 3.46±0.07 b Table 3. The contentof total nitrogen and free amino acidsand the ratio of total sugar to total nitrogenin differenttreatments of '108'
111杨的产卵植株和邻近植株的全N含量均显著低于对照植株,总糖/全N均显著高于对照;产卵植株的游离氨基酸总量显著低于邻近植株和对照植株,且邻近植株与对照的差异不显著(表 4)。
不同处理
Differenttreatments全N
Total nitrogen
/%总糖/全N
Total sugar/
total nitrogen游离氨基酸总量
Total free amino
acids/(mg·g-1)产卵植株
Oviposited plants2.07±0.48 b 9.70±1.22 a 5.24±0.21 b 邻近植株
Neighboring plants2.37±0.42 b 10.54±1.79 a 11.33±0.69 a 对照植株
Control plants3.97±0.05 a 5.67±0.51 b 10.09±0.59 a Table 4. The contentof total nitrogen and free amino acidsand the ratio of total sugar to total nitrogenin differenttreatments of '111'
-
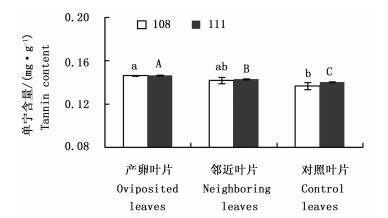
108杨产卵叶片的单宁含量显著高于对照叶片,邻近叶片的单宁含量与对照差异不显著;111杨产卵叶片和邻近叶片的单宁含量均显著高于对照(图 2)。
-
108杨和111杨产卵叶片的总酚含量均显著高于对照,邻近叶片的总酚含量与对照均差异不显著(图 3)。
3.1. 108杨和111杨各处理的幼虫存活率比较
3.2. 108杨和111杨各处理的可溶性糖含量分析
3.3. 108杨和111杨各处理的全N、游离氨基酸总量及总糖/全N分析
3.4. 108杨和111杨各处理的单宁含量分析
3.5. 108杨和111杨各处理的总酚含量分析
-
杨小舟蛾产卵后对产卵植株及邻近植株叶片中的可溶性糖、全N、游离氨基酸、单宁和总酚的含量都有影响,但对2种黑杨派无性系的影响程度不同,其中,108杨的产卵和邻近植株的可溶性总糖含量均显著低于对照,111杨的产卵和邻近植株的二糖含量均显著低于对照,这说明产卵行为的确对自身和邻近植株的可溶性糖含量有影响,而且不同品系杨树在受到害虫侵袭后,体内营养物质会随之发生变化并存在差别,而可溶性糖是昆虫重要的营养物质和能量来源,能刺激昆虫取食[30],该指标含量的降低可能减少了孵化幼虫对糖分的摄入量;同时,可溶性糖含量与树种抗虫性强弱的关系比较复杂。有研究表明,不同小麦品种对玉米象(Sitophilus zeamais(Motschulsky))的抗性[31]、不同种类葡萄对绿盲蝽的抗性指数均与可溶性糖含量相关,含量越低抗性越强[32]。瓜蚜(Aphis gossypii Glover)危害寄主植物后,叶片中可溶性糖含量会显著降低[33];绿盲蝽危害枣树(Zizyphus jujube Mill.)后,叶片内可溶性糖含量随着危害程度的加重先升高后降低[7]。不同程度的机械损失使马尾松(Pinus massoniana Lamb.)针叶内的营养物质(可溶性糖)含量先降低,后逐渐恢复到原来水平[34]。因此,多数寄主植物在受到害虫攻击时,其可溶性糖含量呈现降低的趋势;但也有研究发现,美国白蛾(Hyphantria cunea(Drury))危害白蜡(Fraxinus chinensis Rosb.)、法国梧桐(Platanus orientalis L.)、桑树(Morus alba L.)、臭椿(Ailanthus altissima(Mill.)Swingle)、毛白杨(Populus tomentosa Carr.)和香椿(Toona sinensis(A. Juss.)Roem.)等园林绿化树种后,抗虫性高低与可溶性糖含量没有对应关系,可溶性糖含量的变化没有规律[35]。目前也有研究认为,至少在分子水平上昆虫卵块可能是微生物病原体[36]。病原菌的侵染同样会引起寄主植物营养物质的改变,如黑松(P. thunbergii Parl.)和马尾松接种松材线虫(Bursaphelenchus xylophilus(Steiner et Buhrer))后总糖含量呈不断下降趋势[37]。本实验中,111杨邻近植株的多糖和可溶性总糖含量均显著高于产卵植株,与对照植株差异不显著。所以,邻近植株的诱导抗虫性可能比产卵植株的稍差,或抗虫机制存在差别,还需要进一步的行为测定进行验证。
陈顺立等[38]研究了马尾松针叶营养物质含量与对松突圆蚧(Hemiberlesia pitysophila Takagi)抗性的关系发现,全N和游离氨基酸总量是与抗性关系最密切的指标,含量与抗性呈负相关关系;同时,马尾松高抗家系全N含量低于中抗和低抗家系,并随松突圆蚧的危害而降低,高抗家系降低量大于低抗家系[39]。李镇宇等[40]研究发现,油松(P.tabulaeformis Carr.)受到赤松毛虫(Dendrolimus spectabilis Butler)危害后,受害针叶的氨基酸总量及多数游离氨基酸含量降低。不同棉花品种的蛋白质含量与其对绿盲蝽的抗性存在显著负相关[41]。本研究中,108杨和111杨产卵植株中的全N、游离氨基酸总量均比对照植株的降低,同时2种黑杨派无性系邻近植株的全N含量也均比对照植株的降低,这表明2个品系杨树的产卵和邻近植株都产生了一定的诱导抗性。研究发现,不同水稻品种受到稻飞虱(Nilaparvata lugens Stål)危害后生理指标的变化有差别,受害稻株叶片的可溶性蛋白质、非可溶性蛋白质和蛋白质总量均下降,游离氨基酸含量的变化依品种而不同[42]。108杨和111杨邻近植株的游离氨基酸总量都有所升高,可能是启动了与产卵植株不同的抗性机制。
目前研究发现,植物中的酚类物质与植物抗性存在相互关系。酚类物质是一类分布于植物体中的次生代谢物质,也是植物生长发育必不可少的物质[43]。单宁属于酚类化合物,是植物中一类重要的次生代谢物质和抗虫物质,可以减少植食性昆虫的取食,造成昆虫营养不良、发育缓慢及繁殖力降低[44];单宁还可以抑制消化酶的活性并与淀粉等结合,降低昆虫对营养物质的消化和吸收[45]。瓜蚜危害寄主植物后,叶片中的单宁含量明显上升[33]。杨小舟蛾产卵后2种黑杨派无性系的单宁含量在产卵植株与邻近植株上都有所增加,其中,108杨的产卵植株单宁含量显著高于对照,111杨的产卵和邻近植株都显著高于对照。这与李会平等[46]研究发现不同杨树次生代谢物质的含量差异与天牛危害之间存在明显关系,其中,不同杨树无性系间木质部的单宁含量与其抗虫性呈正相关的研究结果相同。总酚含量在108杨、111杨的产卵叶片、邻近叶片较对照叶片含量有所升高,且108杨和111杨产卵植株的总酚含量均显著高于对照植株。李会平等[46]认为,杨树的酚及酚酸含量越高,对天牛的抗性越强。有研究发现,美洲黑杨不同无性系对分月扇舟蛾(Clostera anastomosis(L.))的抗性大小与叶片中的总酚含量呈明显正相关趋势[47]。因此,本结论说明了杨小舟蛾产卵能够诱导植株自身及相邻植株产生酚类物质,即诱导抗虫性的产生。
本研究表明,杨小舟蛾产卵行为可以在短时间内诱导寄主植物产生抗虫性,本实验各处理叶片是在杨小舟蛾的产卵周期(72h)结束后采集的,说明产卵后的72 h内产卵植株和邻近植株在生理指标上都发生了变化。已有的研究表明,植物能够在较短的时间内启动抗虫系统,拟南芥(Arabidopsis thaliana(L.) Heynh.)在欧洲粉蝶(Pieris brassicae(Linnaeus))产卵后的3 d内改变了包括防御、生长及光合作用在内的上百基因的表达[48];此外,寄主植物在昆虫产完卵后还可通过化感作用吸引天敌,如大豆(Glycine max(Linn.) Merr.)受美洲蝽(Euschistus heros(F.))[49]、榆树(Ulmus campestris L.)在榆叶甲(Xanthogaleruca luteola(Müll.))[50]产卵后其寄生性天敌会在短时间内被寄主挥发物所吸引。本研究已经明确了产卵可以在短时间内诱导寄主植物产生抗虫性,但针对杨小舟蛾产卵后邻近植株与产卵植株间的化感作用还需要深入研究,进一步完善植株间相互作用产生诱导抗性的机制。前人通过野外研究发现,在靠近同种受损个体生长的赤杨(Alnus glutinosa (L.))的被食率较低[51],但植株之间相互诱导产生抗虫性的有效距离却很少有人报道。最新研究发现,菟丝子(Cuscuta australis R.Br.)缠绕的2株较远距离(>100 cm)的同种寄主时,当其中一株受到斜纹夜蛾(Spodoptera litura (F.))幼虫取食时,另外一株的抗虫性会增加[52]。本实验设置邻近植株的距离约50 cm,因此,杨小舟蛾产卵诱导邻近植株产生抗性的距离阈值还有待进一步研究。
另外,用2种黑杨派无性系各处理叶片喂食幼虫后的幼虫存活率均差异不显著,可能是由于扦插苗的叶片较少,叶片被食光后统计存活率不能准确反映最终的存活情况;也可能是幼虫通过自身调节应对植物成分的改变从而减少死亡率。
-
本研究明确了杨小舟蛾在108杨和111杨上产卵后,会导致2种黑杨派无性系的产卵植株和邻近植株叶片中的可溶性糖、全N、游离氨基酸发生不同程度的改变,次生代谢物质单宁和总酚含量都不同程度增加。杨小舟蛾产卵能够诱导108杨和111杨植株自身及相邻植株通过营养物质含量的改变和增加酚类物质含量启动化学防御反应,产生一定的诱导抗虫性。







 DownLoad:
DownLoad: