-
核桃(Juglas regia L.)是核桃科核桃属植物,在植物分类中属于落叶阔叶型高大乔木。体细胞胚(下称:“体胚”)具有数量多、繁殖快、结构完整、植株再生率高以及不受季节影响等特点,是遗传转化的优良试材,也是人工种子制备的基础[1]。自Tuleche等首次报道核桃的体细胞胚发生以来,相继在核桃属的其他种和杂交品种中成功应用[2]。核桃体胚的诱导和发生阶段,外植体与培养基(包含植物生长调节剂)起决定作用。研究表明,DKW培养基是核桃体胚发生的首选培养基,幼胚及其组成成分作为外植体时胚性感受态水平较高[3]。不同果实发育时期的幼胚及其组成成分具有不同的胚性感受态水平,果实发育早期,体胚诱导效果不明显,易产生愈伤组织,而果实发育成熟期,子叶更易于产生不定根[4-6]。前人对采样时间及幼胚形态已有相关报道[7],然而,不同取材时间的形态特征与对应的外观表型的关系还需深入研究。此外,随着胚性愈伤组织培养时间的延长和继代次数的增多,体胚畸变率逐渐升高,再生能力逐渐下降[8]。在核桃的继代培养过程中,通常出现连体胚、子叶畸形胚及玻璃化胚等几种类型的畸形胚,在球形胚阶段人工分离子代体胚或筛选畸形子叶胚可在一定程度降低畸变率,但效率较低[1]。适量浓度的ABA处理不仅能抑制针叶树畸形体胚的产生,而且能提高植株再生率[9]。探索ABA在抑制核桃体胚畸变中的作用,将提高核桃体胚发生及再生效率。在核桃的体胚萌发阶段,存在萌发率较低的问题。低温或者不同干燥处理可提高萌发率。Tuleche等对核桃2℃低温暗培养8~10周后获得核桃的完整植株[2]。Tang等[10]用3~4℃处理了2~3个月的核桃体胚,仅4%~19%萌发为完整植株。张启香等[11]用饱和Ca(NO3)2·4H2O对山核桃体胚进行脱水处理,可将预处理时间缩短至3 d,最高萌发率达39.03%。
早实核桃是我国核桃主栽类型之一。《中国果树志·核桃卷》记载,我国有104个早实核桃类群,尤其以新疆地区较多[12]。早实核桃实生苗的幼龄期为1~3 a,具有结实早、丰产性强等特点[13]。然而,由于其开花年龄提前,树体较小,生命周期较短;同时,早实核桃种子形成过程中胚发育程度也存在差异,在生产中易出现播种出苗率低的现象;在试管继代培养中发现早实核桃容易瓶内开花,从而影响嫩茎的生长[14]。因此,研究早实核桃的体胚发生和再生技术对核桃快繁体系的建立具有重要实践意义,同时也为核桃的基因功能研究奠定了基础。
本研究以‘林早香’(J. Regia)和‘中林6号’(J. regia ‘Zhonglin 6’)花后42~77 d的幼果为试验材料,分别研究了果实发育时期对诱导体胚发生的影响,体胚继代培养过程中ABA对中畸形胚的调节作用以及适度脱水对体胚萌发的促进作用,以期建立高效、稳定的体胚发生和植株再生体系,为早实核桃的工厂化繁育提供实验平台,为早实核桃的遗传转化奠定基础。
HTML
-
‘林早香’为25年生实生大树,种植第2年始果,开花雌性先熟。‘中林6号’为25年嫁接树,砧木为山西小龙眼实生苗,嫁接后第2年始花,开花雄性先熟。2种核桃幼果均采自中国林业科学研究院图书馆前。采集时间为2016年5月28日至6月25日,即盛花期后42~70 d,每7 d采集1次。
-
将未成熟果实的外果皮用洗洁剂刷洗干净,在超净台内进行表面消毒和接种工作:将冲洗干净的核桃青果置于无菌的大烧杯内,75%的酒精消毒1 min,无菌水冲洗3~5次,之后浸没于2%NaClO,抽真空处理20 min,无菌水漂洗3~5次,之后用无菌的修枝剪剖开,用无菌镊子将幼胚与胚乳分离,幼胚用于接种。
-
分别将‘林早香’和‘中林6号’的幼胚接种于DKW+2.0 mg·L-1 BA+30 g蔗糖固体培养基中暗培养,温度为(23±2)℃,每个胚龄接种3个平板,每个平板15个幼胚。3周后观察并统计不同发育时期幼胚的体胚诱导率。
-
统计继代培养20代和40代的体胚发生畸变率,以继代40代相同状态的成熟体胚为材料,在含有不同浓度ABA(0.0、0.5、1.0、2.0、4.0 mg·L-1)的DKW培养基处理10 d,转置不含激素的DKW培养基上继代培养,观察子代体胚的形态并统计体胚发生率及畸变率。每个处理重复3次,每个处理接种15个幼胚,3周后统计体胚的畸变率。
-
将成熟体胚用饱和NH4NO3溶液环境(23℃,暗培养)进行脱水处理,分别处理0、24、48、72、96、120 h,转置于DKW+1.0 mg·L-1 6-BA+0.01 mg·L-1 IBA+30 g蔗糖固体培养基上,(25±2)℃、16 h·d-1光照和40 μmol·m-2·s-1光强的组培室中培养。每个处理30个待萌发的体胚,重复3次,6周后统计失水率和萌发率。
-
体胚诱导率=(诱导出体胚的外植体数/接种的外植体数)×100%,体胚畸变率=(畸形体胚个数/体胚总个数)×100%,体胚萌发率=(萌发的体胚数/脱水处理的体胚数)×100%。
1.1. 试验材料
1.2. 试验方法
1.2.1. 外植体的消毒
1.2.2. 早实核桃体胚的诱导
1.2.3. 早实核桃体胚的继代培养
1.2.4. 早实核桃体胚的脱水及萌发
1.2.5. 数据处理
-
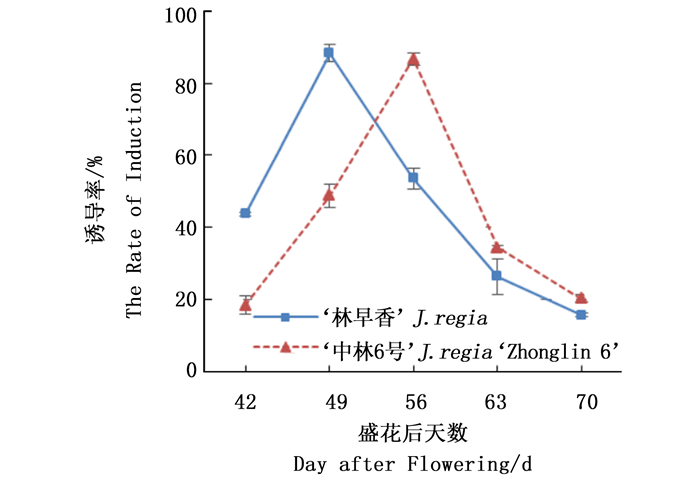
核桃体胚发生与幼果的发育时期有密切关系,不同发育时期的幼果诱导产生体细胞胚的能力存在显著差异。随着‘林早香’和‘中林6号’幼果的生长,体胚诱导率呈先升高后降低的趋势,分别于花后约49 d和56 d达到最高,最大诱导率分别为88.32%±2.28%和86.67%±1.57%(图 1)。
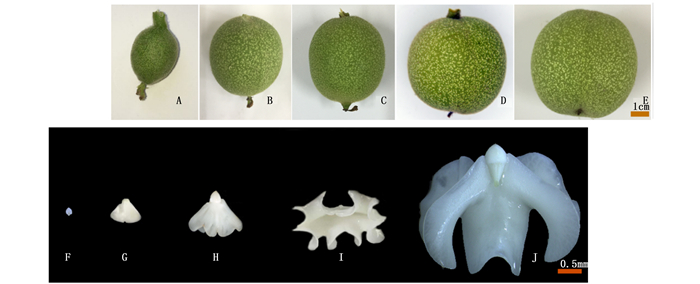
不同发育时期幼果的外观表型也不同。‘中林6号’幼果发育比‘林早香’晚7 d左右。图 2所示,‘中林6号’5个采样时期的幼果,花后42 d,果实膨大,果皮密被黄绿色柔毛,果顶渐尖,柱头和雌蕊未脱落,种室内胚乳胶状,部分果实出现球形胚或鱼雷胚;花后49 d,果皮密被柔毛变少,果顶变圆,柱头枯萎未脱落,幼胚为乳白色翅状;花后56 d,果实迅速膨大,果皮变光滑,果壳变硬,子叶伸展,柱头逐渐脱落;花后63 d,子叶迅速伸展,出现多个内褶;花后72 d,子叶充满种室,柱头完全脱落。
-
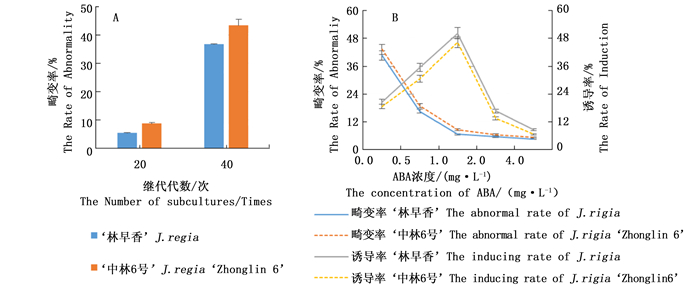
随着继代培养次数的增多,子代体胚出现大量畸形胚,从而影响体胚的萌发。‘中林6号’继代20代时,体胚的畸变率约为8.67%,继代培养40代时,畸变率上升到约43.33%,而‘林早香’畸变率略低(图 3A)。适宜浓度的ABA可提高体胚诱导率并降低体胚畸变率。随着ABA浓度的增加,体胚畸变率呈下降趋势,ABA浓度>1.0 mg·L-1时,ABA的调节效应不明显;而体胚诱导率呈现先升高后降低的趋势,ABA浓度>1.0 mg·L-1时,体胚子叶干皱,呈现老化状态,体胚诱导率降低(图 3B)。因此,最佳ABA调节浓度为1.0 mg·L-1。
-
随着成熟体胚脱水时间的延长,体胚萌发率呈现先升高后降低的趋势(表 1)。脱水处理0~24 h的体胚,胚根伸长,但难成苗;脱水处理48 h‘中林6号’和‘林早香’的体胚萌发率显著提高,分别为16.67%±0.80%和18.82%±0.22%;脱水72 h后,‘中林6号’和‘林早香’体胚萌发率最高,分别为53.33%±0.13%和56.67%±0.39%;若干燥时间继续延长,体胚的萌发率下降,部分体胚的子叶和生长点焦枯,甚至坏死,影响体胚的萌发。
处理
Treament脱水时间/h
Desiccation length萌发率/%
Germination percentage‘林早香’ ‘中林6号’ 1(对照Control) 0 6.67±0.06 F 3.36±0.12 e 2 24 8.78±0.12 D 3.34±0.05 e 3 48 18.82±0.22 C 16.67±0.80 c 4 72 56.67±0.39 A 53.33±0.13 a 5 96 35.56±0.14 B 35.56±0.20 b 6 120 7.12±0.13 E 6.67±0.07 d Table 1. Effect of deciccation on germination of two precocious walnuts
将脱水处理后的体胚置于2.0 mg·L-16-BA+0.01 mg·L-1 IBA的固体培养基进行光照培养,4~5 d之后胚根伸长,茎端生长点变为绿色(图 3A),1个月后可发育成完整植株(图 3B)。
2.1. 早实核桃幼果发育时期对体胚发生的影响
2.2. ABA对抑制畸形胚的调节作用
2.3. 脱水处理对早实核桃体胚萌发的影响
-
植物外植体的组织、基因型及发育时期等都对体胚的诱导产生影响。在核桃中,幼胚及子叶具有较高的胚性感受态水平。本研究采用早实核桃幼胚作外植体获得核桃体胚,2种早实核桃体胚诱导率不同,‘林早香’比‘中林6号’略高,表明早实核桃幼胚的发育时期对其胚性感受态水平的影响至关重要。资料表明,Payne、Early Ehrhardt、Franquette、Scharsch-Franquette和Plant Introduction 18256等核桃的5个栽培品种的体胚最佳诱导时间不同,分别为花后7~11周[2]。山核桃8~10周的合子胚最易形成愈伤组织,间接发生体细胞胚;而11~12周的合子胚可直接诱导发生体细胞胚[15]。黑核桃在授粉后6~7周时子叶的体胚发生能力最高。这可能与核桃果实成熟的一个关键时期有关系,在该时期子叶迅速生长,果壳变为半木质化[7]。本研究中,随着‘林早香’和‘中林6号’分别于花后约49 d和56 d时幼胚的体胚诱导率最高,此时果实的表型特征为果实状态为外果皮绒毛变少,开始变光滑,种壳近胚端变硬,子叶迅速伸展,胚乳液体状。本研究将为早实核桃体胚发生体系的建立提供理论指导。
-
畸形胚在植物体胚发生过程中普遍存在,主要表现为连体胚、子叶畸形胚、重新愈伤化的体胚、玻璃化体胚及生长点畸形胚[16]。畸形胚不能发育成苗,严重影响体胚在生产和育种中的应用。通过研究橡树体胚发生各个时期的内源激素变化,结果表明,ABA在橡树E2时期内源含量最高[17]。在继代培养基中加入适量ABA能抑制核桃楸畸形胚的产生[18]。这可能是由于外源ABA促进了细胞蛋白的积累,抑制细胞扩增并促进体胚的发育[19]。ABA在激素水平提高了体胚的耐干旱性,从而提高体胚的质量[20]。本研究中,2种早实核桃子代培养40代后,产生大量畸形体胚,畸变率分别为36.67%和43.33%,添加低浓度ABA(1.0 mg·L-1)不仅降低了体胚畸变率,且在一定程度上提高了早实核桃的体胚发生率。
-
核桃体胚的成熟、萌发及成株率较低是限制其应用的主要问题。正常体胚子叶乳白色且有生长点,成熟后,经过低温或脱水处理后可大大缩短萌发时间,提高萌发率,这可能是由于体胚进入与自然种子相似的休眠和静止状态[21]。Jariteh等对Persian核桃合子胚和体胚成熟过程中进行研究,发现成熟的合子胚及体胚中含有较高浓度的脯氨酸、蛋白及抗氧化酶活性[22]。相较于低温(2~4℃)处理,脱水处理时间变短,常用的干燥剂有CaCl2·6H2O、Ca(NO3)2·4H2O、ZnSO4及NH4NO3饱和溶液。本研究用饱和NH4NO3溶液对成熟体胚进行不同天数脱水处理,结果表明,3 d脱水处理下体胚失水量约为40%,此时体胚萌发率达到最高。该处理为早实核桃的植株再生及工厂化生产提供了良好的技术支持。
3.1. 影响早实核桃体胚发生的关键因素分析
3.1.1. 果实不同发育时期对核桃体细胞胚发生的影响
3.1.2. ABA对继代培养的调节作用研究
3.2. 早实核桃体胚植株再生过程的分析
-
在早实核桃‘中林6号’和‘林早香’的继代培养过程中,适度ABA处理可提高继代胚的质量。体胚萌发前,进行脱水处理可大大提高体胚萌发率。早实核桃体胚发生和植株再生体系的建立,完善了核桃属快速繁殖技术,也为其他物种的相关研究提供了参考依据。








 DownLoad:
DownLoad: