-
松材线虫病,即松树枯萎病(pine wilt disease, PWD),是一种以松材线虫(Bursaphelenchus xylophilus)为病原的国际检疫性林业病害[1-2]。松材线虫病具有发病部位隐秘、发病速度快、传播蔓延迅速、传播途径多、治理难度大等特点,因此该病害又被称为松树的“癌症”[3]。我国于1982年在南京中山陵首次发现松材线虫病[4],随后在我国逐渐蔓延,已经造成了重大的经济损失并严重威胁着我国众多名胜风景区和松林生态系统安全。细胞色素P450(cytochrome p450, P450),又称单加氧酶,是一类由数量众多、功能复杂的血红素结合蛋白组成的同工酶,广泛存在于细菌、真菌、动物和植物等生物体中[5]。细胞色素P450在内源性化合物(脂肪酸、胆汁酸、类固醇、蜕皮激素和保幼激素等)和外源性化合物(农药、植物毒素和环境致癌物等)代谢过程中十分重要[6-8]。大量研究表明细胞色素P450基因参与代谢过程和抗性机制主要通过上调来实现。Maja等[9]研究发现秀丽隐杆线虫受到毒物质侵害时,cyp-33D3基因表达量上调明显。松材线虫全基因组序列于2011年测序完成,为研究松材线虫致病相关基因的功能奠定了良好的基础[10]。我们前期研究发现松材线虫感染松树后,cytochrome P450 33D3 (cyp-33D3) 基因表达量上调显著,表明cyp-33D3基因在松材线虫致病过程中发挥了重要作用[11]。目前关于cyp-33D3基因的研究主要集中在过量表达方面,而关于该基因的功能研究鲜见报道。
RNA干扰(RNA interference, RNAi)现象广泛存在于真核生物细胞中,它通过小分子双链RNA诱导同源mRNA降解从而阻断体内特定基因表达[12]。随着RNA干扰机制被阐明,RNA干扰被认为是一种可以与基因敲除相媲美的技术,在基因功能、信号传导通路和基因治疗等研究领域得到了广泛的应用[13-16]。本研究采用RNA干扰技术研究cyp-33D3基因沉默后对松材线虫取食、繁殖和致病性的影响,阐明cyp-33D3基因在松材线虫致病过程中的功能,为进一步明确松材线虫分子致病机制奠定理论基础。
HTML
-
供试松材线虫BxJJ01分离自江西省九江市的黑松疫木,并保存于九江学院植物基因资源重点实验室。将松材线虫接种至长满灰葡萄孢(Botrytis cinerea)的马铃薯葡萄糖琼脂(potato dextrose agar, PDA)平板培养基上,25℃恒温培养5 d,采用贝尔曼漏斗法分离线虫,用无菌水清洗3次,1500 r·min−1离心5 min。将离心得到的线虫悬浮液储存于1.5 mL离心管中,备用。
-
根据RNA抽提试剂盒(天根生化科技有限公司)的使用说明书提取松材线虫总RNA。利用紫外分光光度计(Nanodrop 2000)测定RNA浓度,通过1%琼脂糖凝胶电泳检测RNA的质量。根据TaKaRa公司反转录试剂盒(Prime Script 1st strand cDNA Synthesis Kit)使用说明书将松材线虫总RNA反转录为cDNA用于后续PCR实验。
-
依据GenBank上下载的cyp-33D3(GenBank: KM973212.2)基因序列设计上游引物cyp-33D3-F(5′-TCACCGACTACGAGGTCC-3′)和下游引物cyp-33D3-R(5′-ATGGGATGAACGACGAAC-3′),以合成的cDNA为模板进行PCR扩增。反应条件为:94℃预变性5 s,94℃变性30 s,60℃34 s,72℃1 min,35个循环,72℃延伸10 min。根据Axygen公司的切胶回收试剂盒使用说明书对目标片段进行切胶回收,并克隆于PmdTM18-T载体上,转化至大肠杆菌感受态细胞,委托华大基因公司进行测序。
-
以PmdTM18-T和pCH-sGFP载体为模板,通过带有T7启动子的引物进行PCR扩增,采用MEGAscript RNAi Kit Protocol 试剂盒(Life Technologies 公司)分别合成松材线虫cyp-33D3和gfp基因dsRNA。用无RNA酶H2O适当稀释合成的dsRNA,通过紫外分光光度计测定A260值,同时测定溶解dsRNA相应H2O的A260值作为空白值,dsRNA浓度(μg·mL–1)为260 nm的吸光值减去空白值后乘以稀释倍数再乘以系数40。经测定合成的cyp-33D3和gfp基因dsRNA浓度分别为1.45 mg·mL–1和1.53 mg·mL–1,用1%琼脂糖凝胶电泳检测dsRNA完整性,将合成好的dsRNA储存于–80℃冰箱,备用。
dsRNA浓度(μg·mL–1)=(A260−空白值) × 稀释倍数 × 40
-
将收集的松材线虫混合虫态浸泡于1 μg·μL–1 的cyp-33D3 dsRNA中[17],以gfp dsRNA 作为非靶标性dsRNA对照,以ddH2O作为空白对照,每个处理约8000条线虫,每次试验3次重复,将各处理置于25℃摇床170 r·min−1 48 h后[15-16],用ddH2O洗脱线虫。
分别挑取30对(成熟雄虫和雌虫各30条)干扰后和对照组的松材线虫,接种至长有灰葡萄孢的PDA平板上,25℃条件下培养5 d,用贝尔曼漏斗法进行分离。拍照观察各处理松材线虫取食情况,用光学显微镜统计其数量,每个处理3次重复。
分别挑取20对(成熟雄虫20条,怀孕雌虫20条)干扰后和对照组的松材线虫,置于直径为30 mm的小培养皿上,加入适量ddH2O,25℃黑暗培养12 h后,轻轻倒掉线虫悬浮液,用无菌水将粘附在培养皿底部的线虫虫卵洗出,收集松材线虫卵粒并统计其数量,每个处理重复3次。
分别挑取100颗线虫虫卵浸泡在等量的ddH2O、gfp dsRNA和cyp-33D3 dsRNA溶液中,置于25℃恒温培养箱黑暗条件下孵化48 h。使用倒置显微镜对已孵化卵的数量进行计数,每个处理重复3次。
分别挑取20对(成熟雄虫和雌虫各20条)干扰后和对照组的松材线虫,接种至长有灰葡萄孢的PDA平板上进行交配和繁殖,从而得到子一代(F1)的老熟成虫。将获得的成虫转移至孔板并用高温杀死,在光学显微镜下测量其个体长度,每个处理重复3次。
采用人工皮接法分别将ddH2O浸泡和cyp-33D3 dsRNA干扰后含有2 000条松材线虫的悬液接种到生长情况一致的两年生黑松上,同时将等体积的gfp dsRNA溶液接种至黑松,每个处理接种6株。接种后观察松树的发病情况,持续观察40 d,统计发病率。
-
分别提取对照组和干扰后子一代(F1)松材线虫总RNA,反转录合成第1链cDNA,以松材线虫Actin基因为内参基因,根据TransStart Green qPCR SuperMix(北京全式金生物技术有限公司)试剂盒说明书进行荧光定量PCR,测定松材线虫cyp-33D3基因的表达量。
1.1. 实验材料
1.2. 松材线虫总RNA提取及cDNA的合成
1.3. 松材线虫cyp-33D3基因片段的克隆
1.4. 松材线虫cyp-33D3基因dsRNA的合成
1.5. 松材线虫cyp-33D3基因的RNA干扰
1.6. 松材线虫cyp-33D3基因干扰效率的测定
-
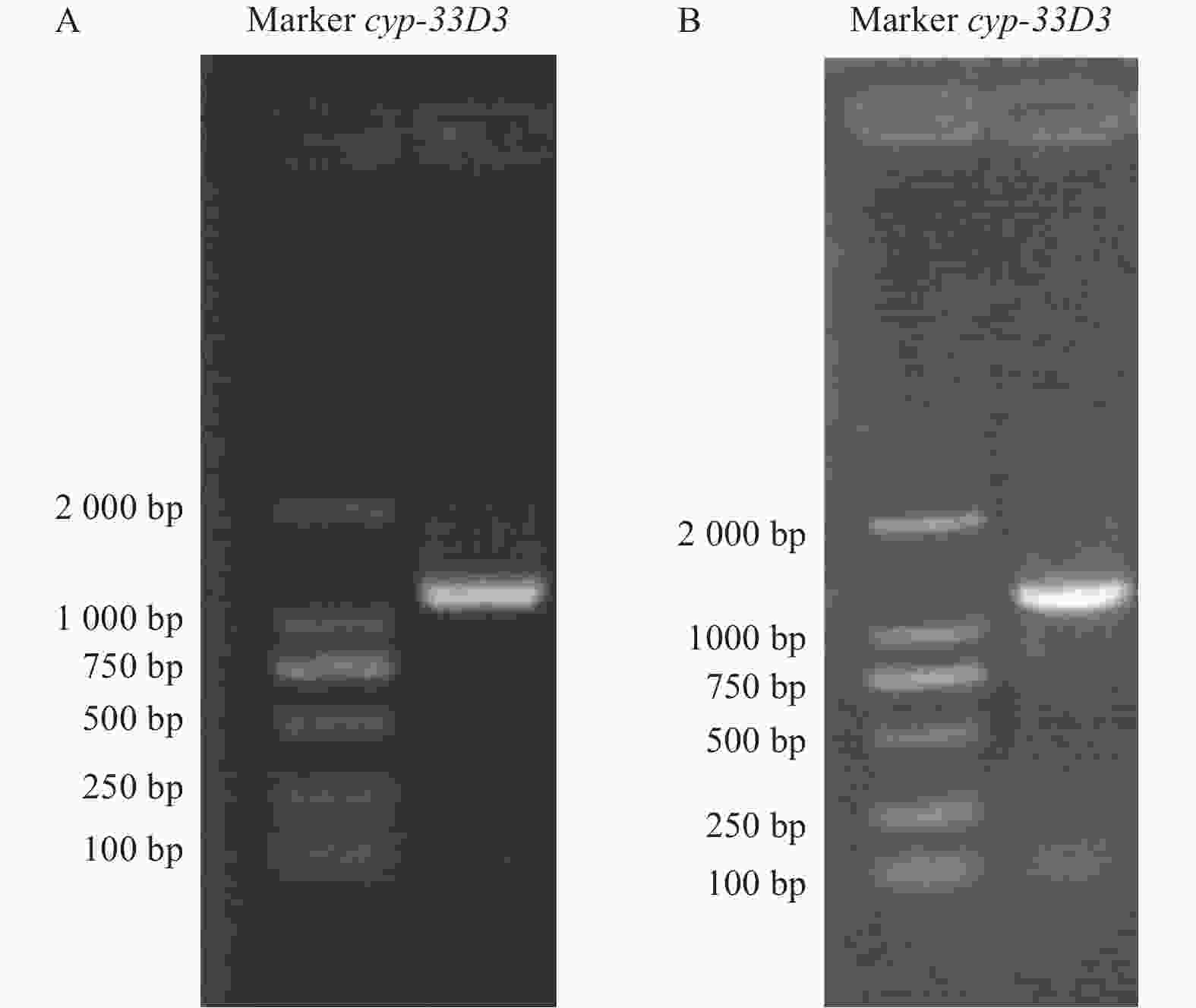
以松材线虫cDNA为模板,用特异性引物扩增获得细胞色素cyp-33D3基因,结果显示扩增片段长度介于1000~1500 bp之间(图1A),与预期大小1192 bp相符。回收目的条带与pMDTM19-T载体连接后转化至大肠杆菌Trans1-T1感受态细胞中,挑取阳性克隆进行测序验证。通过带有T7启动子的特异性引物进行PCR扩增,合成dsRNA。从图1B中可以看出,琼脂糖凝胶电泳条带单一、明亮、完整,可用于后续的RNA干扰实验。
-
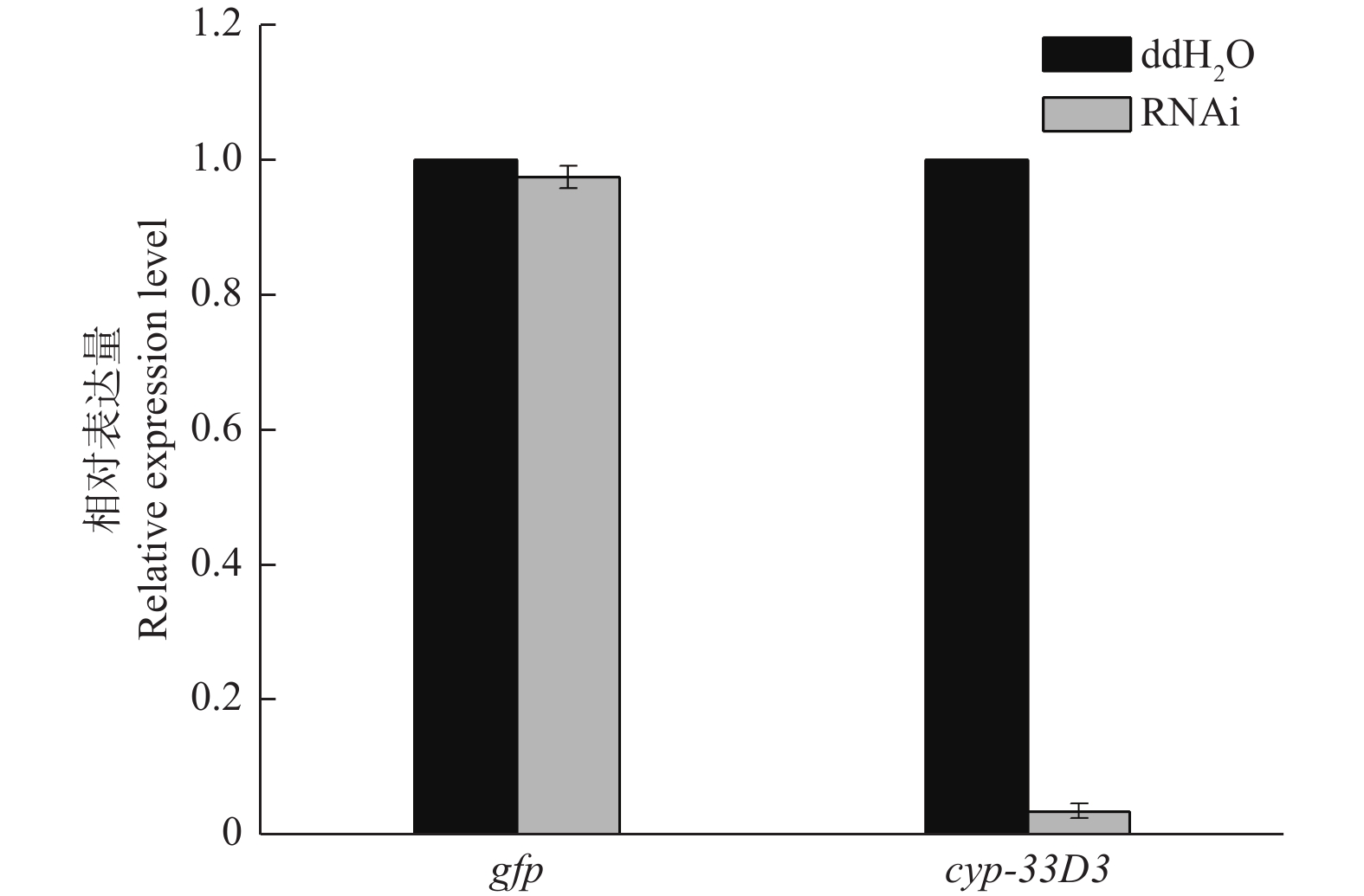
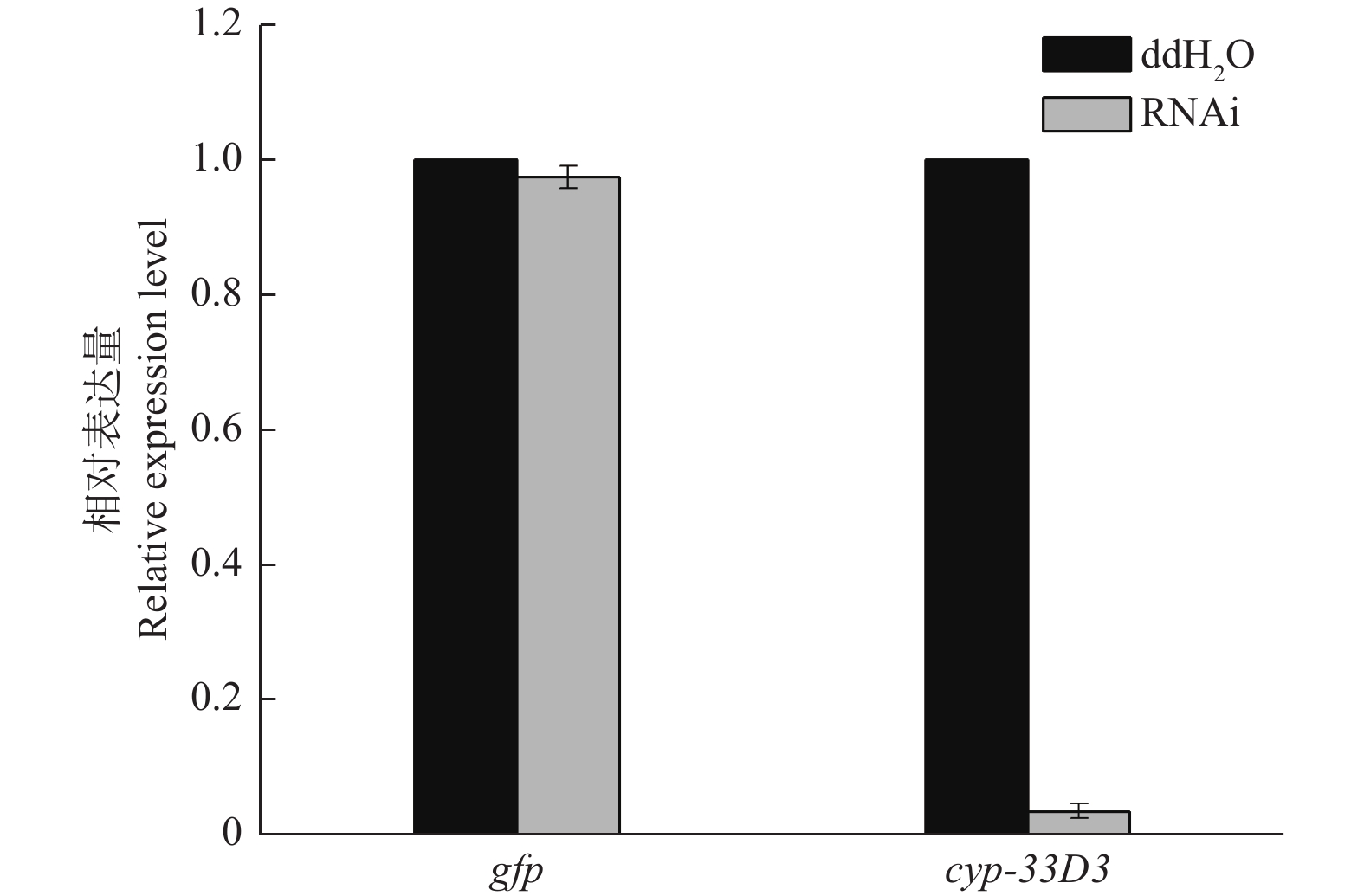
如图2所示,松材线虫经gfp dsRNA和cyp-33D3 dsRNA浸泡后,cyp-33D3基因的表达量分别为0.974和0.036(将ddH2O处理对照组的细胞色素cyp-33D3基因表达量设为1),说明外源dsRNA对松材线虫细胞色素cyp-33D3基因表达量无影响,而cyp-33D3 dsRNA可以有效抑制靶基因的表达。
-
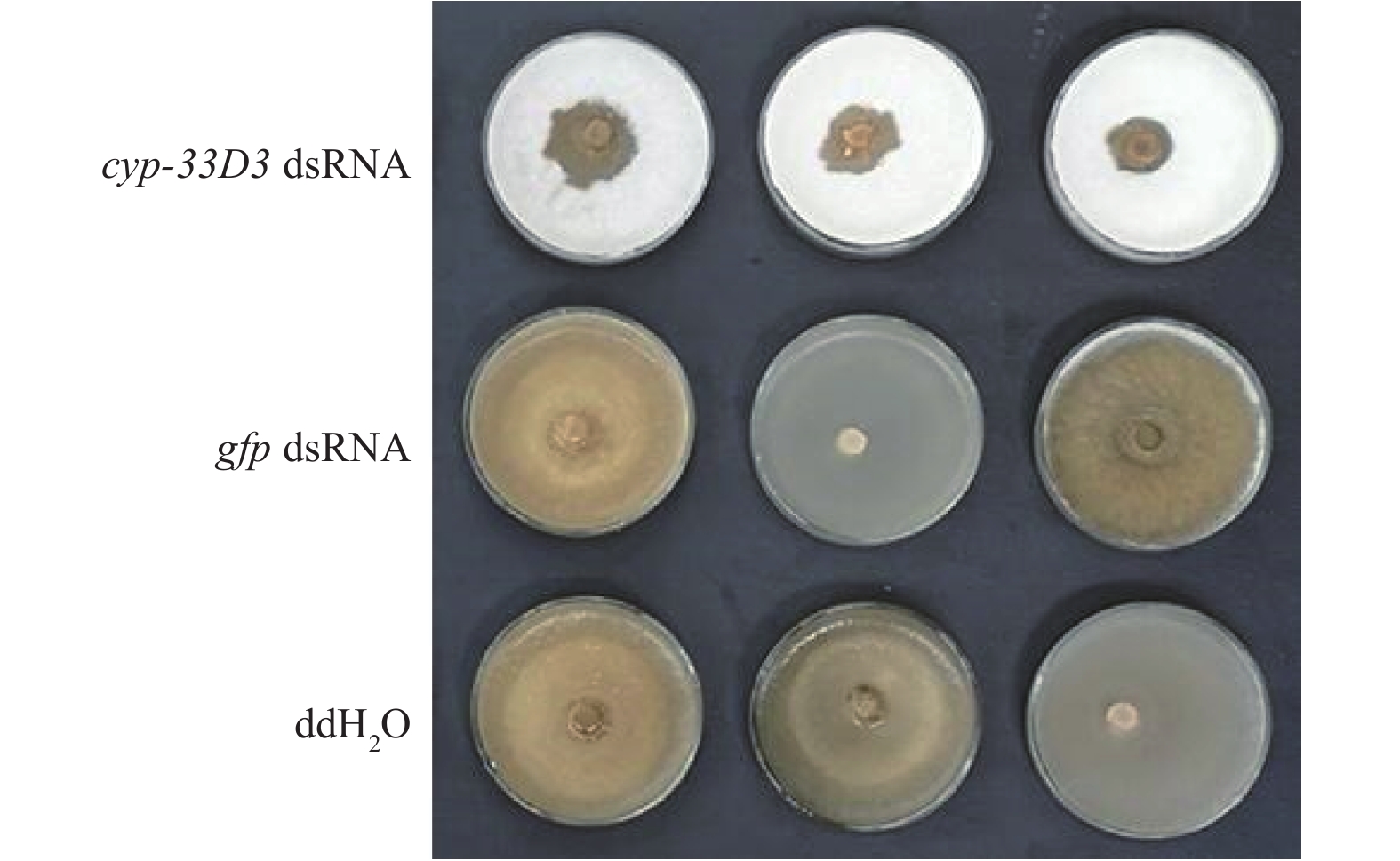
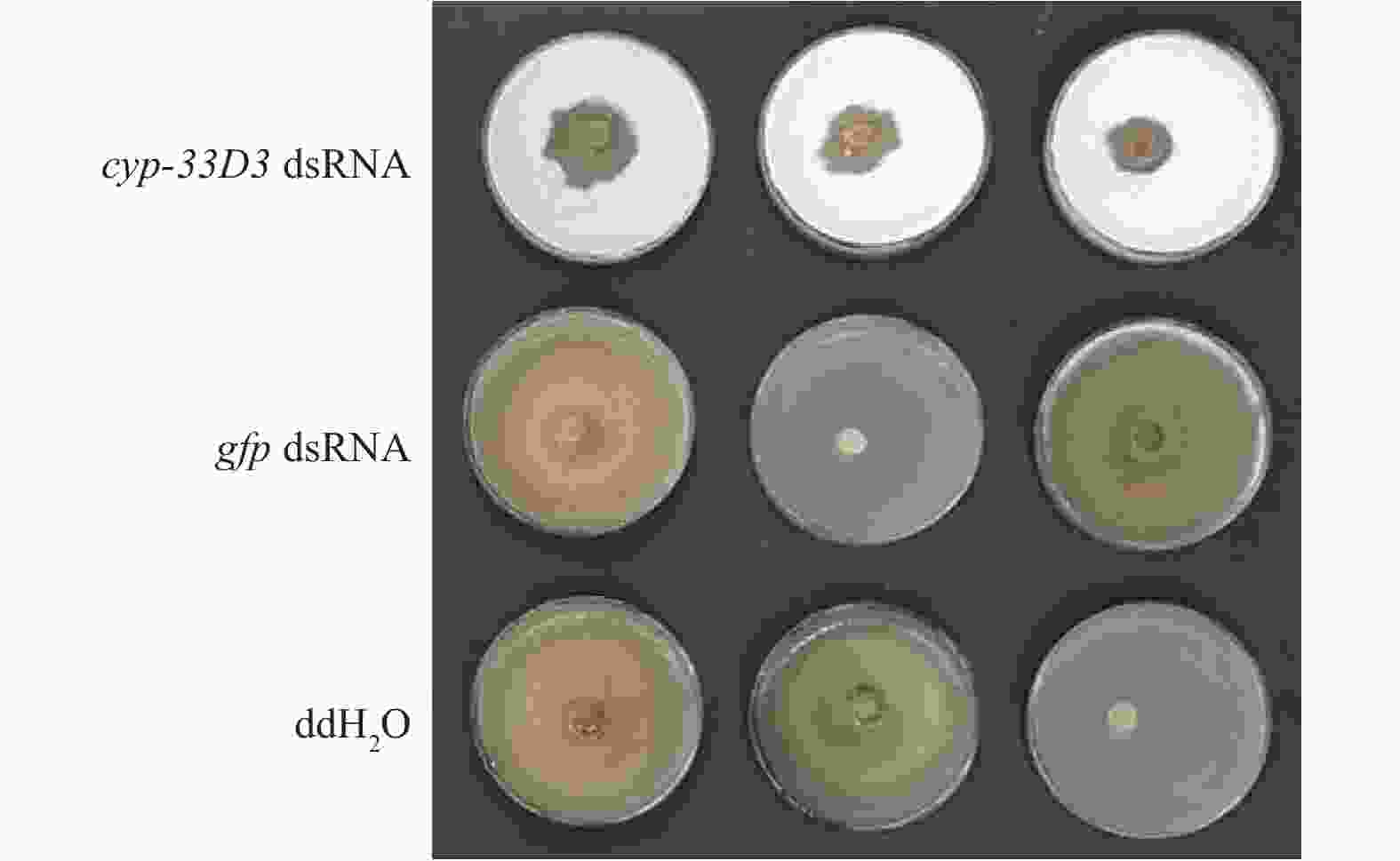
从图3可以看出,ddH2O和gfp dsRNA处理的线虫取食速度明显较快,在第5天已经几乎将灰葡萄孢菌丝取食殆尽,而cyp-33D3 dsRNA处理松材线虫取食灰葡萄孢的面积较小,说明细胞色素cyp-33D3基因表达沉默后对松材线虫的取食有重要影响。
-
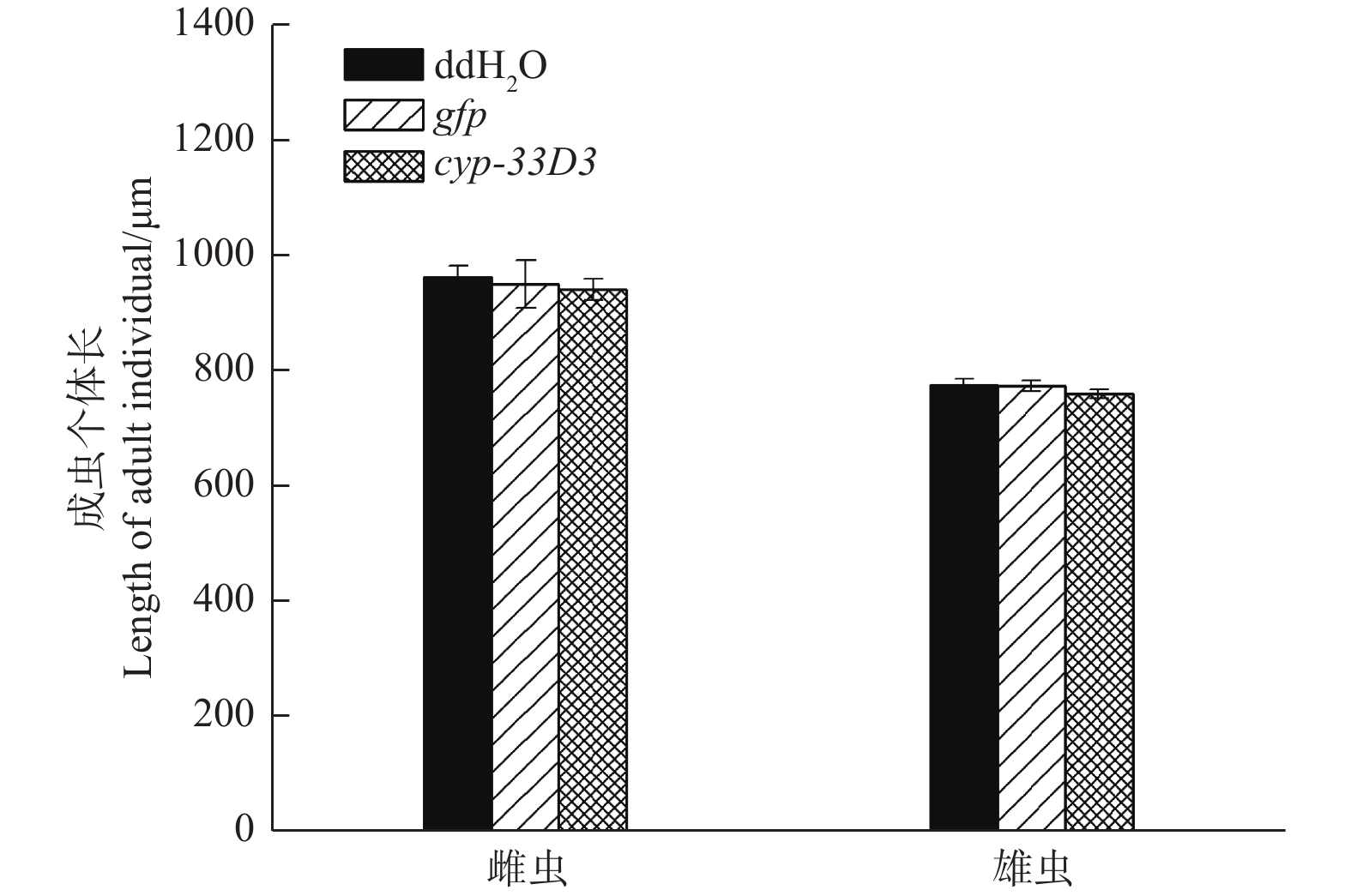
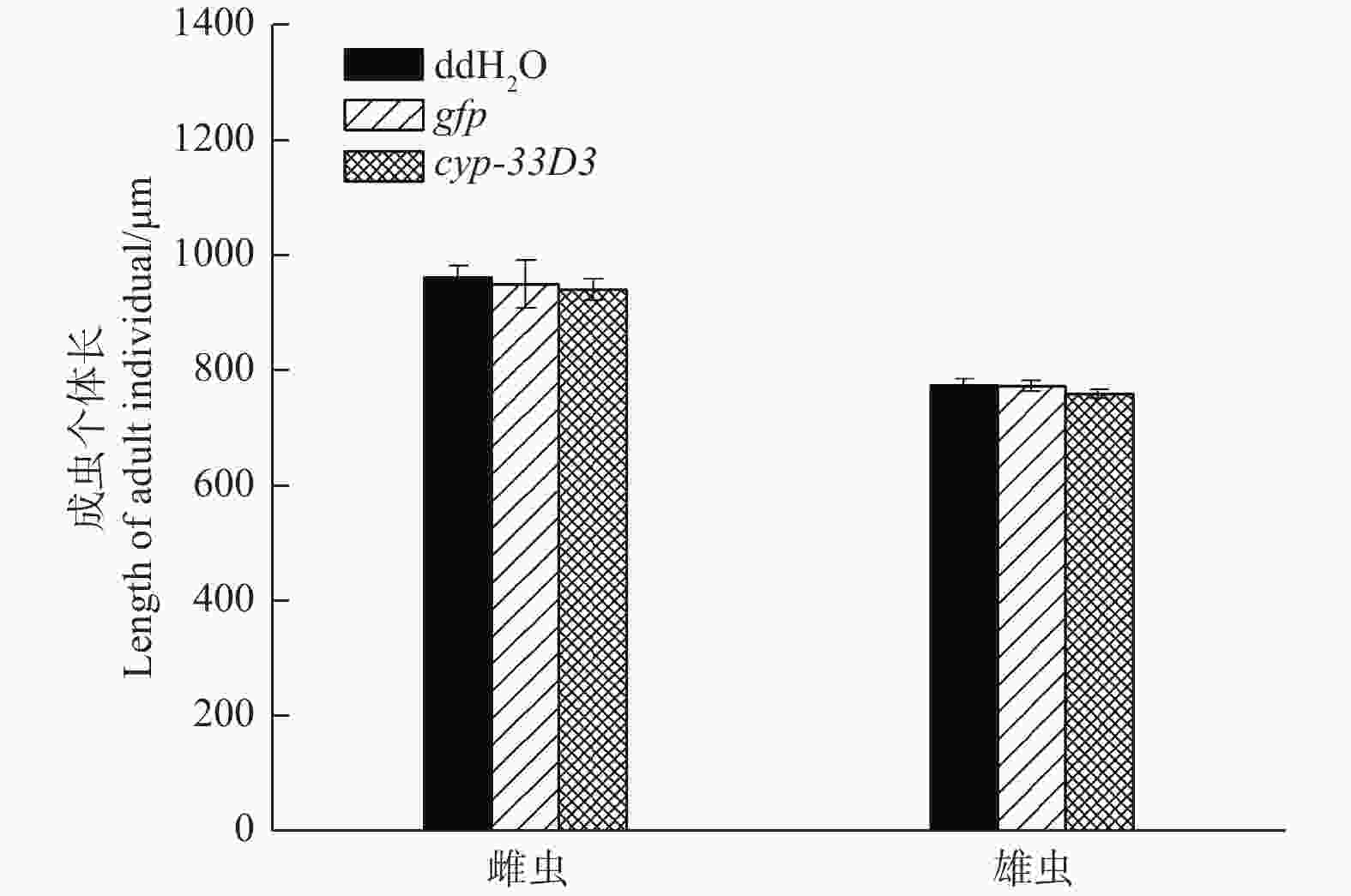
经测量,ddH2O处理雌、雄成虫的体长分别为964.33 μm和777.12 μm;gfp dsRNA处理雌、雄成虫的体长分别为951.66 μm和775.34 μm;cyp-33D3 dsRNA处理雌、雄成虫的体长分别为942.57 μm和761.33 μm(图4)。RNA干扰后雌、雄成虫的体长有所减短,但差异不显著(P > 0.05)。因此,我们推测RNA干扰对松材线虫体长无明显影响。
-
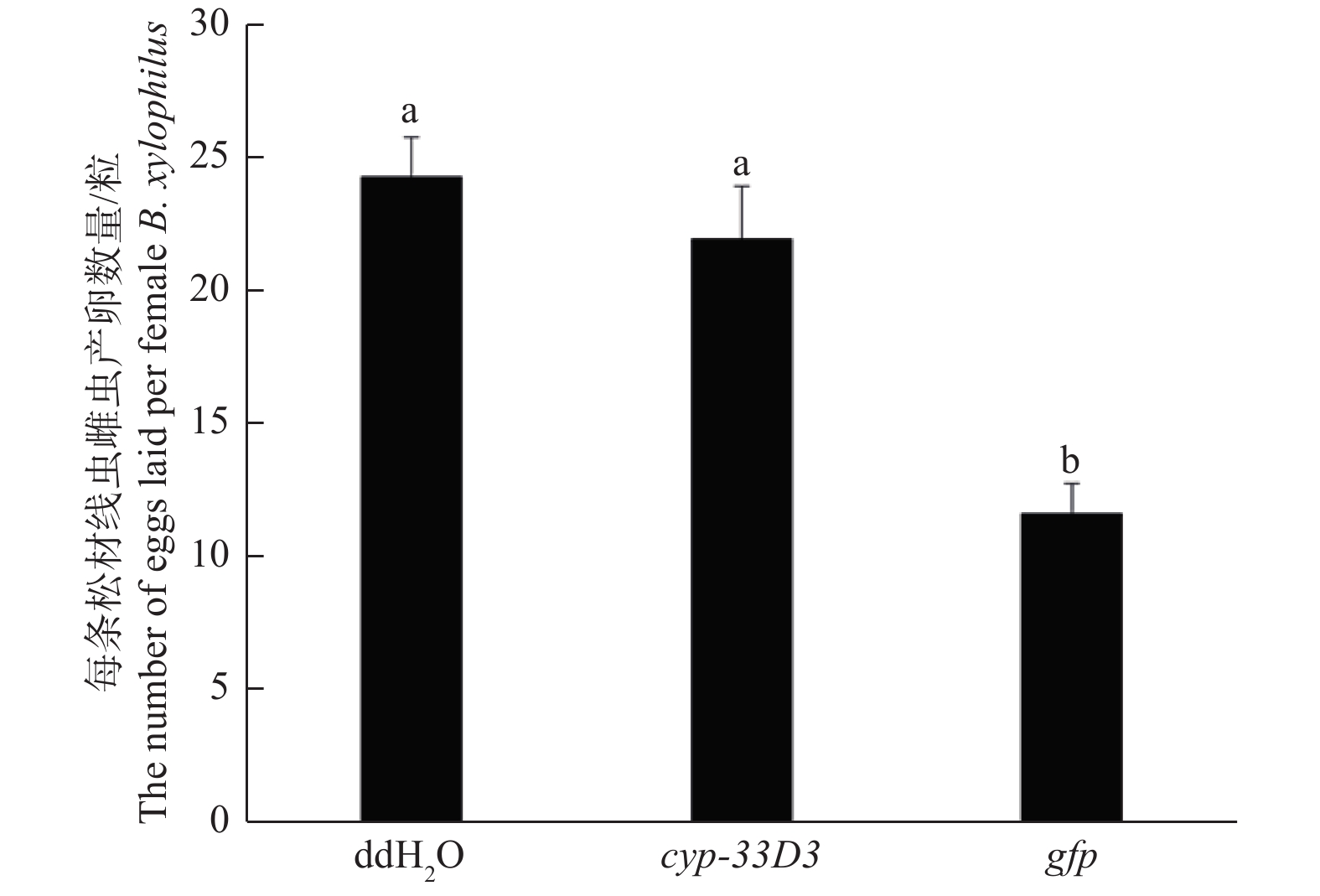
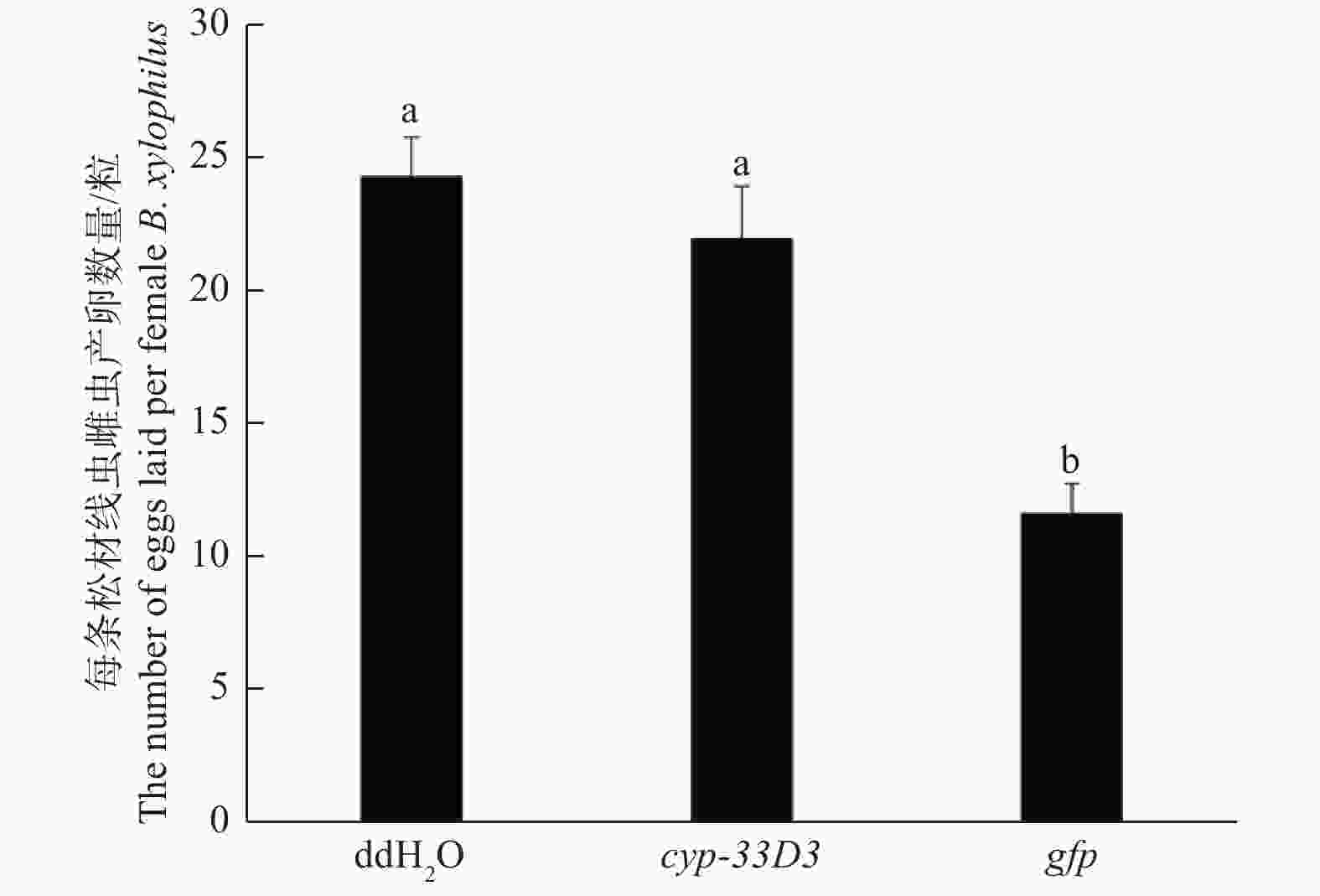
由图5可知,ddH2O和gfp dsRNA处理的每条雌虫平均产卵数量分别为24和23粒,而cyp-33D3 dsRNA处理中每条雌虫平均产卵数量为12粒。结果表明,cyp-33D3 dsRNA干扰显著(P < 0.01)减少了雌虫的产卵数量,降低了松材线虫的繁殖能力。
-
不同处理的松材线虫卵孵化率如图6所示,cyp-33D3 dsRNA处理的虫卵孵化率为49.67%,与ddH2O处理相比差异显著(P < 0.01);而gfp dsRNA处理和ddH2O处理的虫卵孵化率无明显差异(P > 0.05)。由此可见,cyp-33D3 dsRNA干扰对松材线虫卵孵化具有一定的抑制作用,而非内源双链RNA干扰对其无影响。
-
在接种40 d后,ddH2O处理的黑松针叶全部枯萎,发病率达到100%;cyp-33D3 dsRNA处理的黑松发病率为43.1%;而gfp dsRNA处理的黑松在整个实验过程中生长状况良好,发病率为零(图7)。据此推测,RNA干扰在一定程度上减弱了松材线虫的致病能力,进而降低了黑松的发病率。
2.1. 松材线虫cyp-33D3基因的克隆
2.2. 干扰后松材线虫cyp-33D3基因表达量分析
2.3. 松材线虫cyp-33D3基因RNAi对线虫取食的影响
2.4. 松材线虫cyp-33D3基因RNAi对线虫个体体长的影响
2.5. 松材线虫cyp-33D3基因RNAi对线虫产卵的影响
2.6. 松材线虫cyp-33D3基因RNAi对线虫孵化率的影响
2.7. 松材线虫cyp-33D3基因RNAi对线虫致病力的影响
-
由于松材线虫的生活史和寄生环境与其他线虫差别很大,致使其分子致病机制研究进展缓慢。Kikuchi等人于2011年完成了松材线虫全基因组测序,这为进一步研究松材线虫致病基因的功能奠定了良好的基础。松材线虫感染寄主后,寄主会分泌一系列的次级代谢产物抵御松材线虫的入侵。有研究表明,CYP450基因产物是松材线虫代谢寄主松树次级代谢产物第一阶段最重要的酶。我们的前期研究结果发现在松材线虫入侵寄主后cyp-33D3基因表达量上调显著,说明该基因在松材线虫致病过程中发挥了重要作用。
RNA干扰是由与靶基因序列同源的双链RNA引发的广泛存在于生物体内的序列特异性基因转录后沉默的过程。RNA干扰具有特异、有效的基因沉默效应,克服了建立基因敲除动物模型费时费力的缺点,已成为研究基因功能非常有效的手段。Wang等[18]通过RNA干扰技术研究松材线虫精氨酸激酶(BxAK1)基因的功能,发现该基因表达量下调增加了线虫的死亡率,同时减弱了线虫的繁殖能力。Kang等[19]通过该方法对松材线虫毒液过敏原样蛋白(BxVap-1)基因进行RNA干扰,结果表明毒液过敏原样蛋白基因与线虫的迁移能力有密切联系。魏丽莎子等[20]利用RNA干扰技术对象耳豆根结线虫二龄幼虫Me-mapk1基因进行沉默,线虫诱导番茄根结形成的数量显著减少,推测Me-mapk1基因的下调表达有可能与象耳豆根结线虫二龄幼虫(J2)的生长发育相关。以上研究表明,RNA干扰是一种研究基因功能的有效技术。细胞色素P450作为线虫体内三大与代谢抗性相关的超基因家族之一,对松材线虫生长发育和致病性起到至关重要的作用。Benenati 等[21]通过RNA干扰技术研究了秀丽隐杆线虫(Caenorhabditis elegans)cyp-31A2和cyp-31A3基因的功能,发现这两个基因表达量下调导致线虫胚胎细胞不能正确执行减数分裂以及卵壳形成所需的脂质物质合成受阻。Verma等[22]研究表明,cyp5122A1基因表达量下降增加了杜氏利什曼虫(Leishmania donovani)的死亡率,降低了利什曼虫对药物的敏感性和减少了麦角固醇的合成。本研究通过RNA干扰技术沉默了松材线虫cyp-33D3基因,发现cyp-33D3基因下调表达后,松材线虫取食速率降低,繁殖能力下降。这可能是由于cyp-33D3基因沉默后影响了松材线虫胚胎细胞减数分裂的正确执行,抑制了卵壳形成所需脂质物质的合成以及增加了线虫的死亡率,导致线虫繁殖数量下降,进而影响了松材线虫的取食速度。
植物寄生线虫致病的前提是能够在寄主体内存活和进行繁殖。我们将cyp-33D3 dsRNA干扰后的松材线虫接种至黑松苗上,发现线虫的致病能力明显下降,推测原因可能是:(1)cyp-33D3基因表达沉默后,松材线虫在松树体内的取食和产卵受到影响,进而降低了线虫的繁殖数量,导致其致病力下降;(2)松材线虫入侵寄主后,松树会分泌出大量的次生代谢产物抵御松材线虫的入侵,松材线虫为了应对这些次生代谢产物的毒害作用,必须通过解毒基因对该类物质进行代谢转化,由于CYP450基因在松材线虫解毒过程中具有重要作用,因此cyp-33D3基因被表达被沉默后,可能致使松材线虫不能有效降解寄主分泌的有毒次生代谢产物,大量线虫被杀死,最终导致松材线虫的致病力下降。我们的研究结果表明cyp-33D3基因与松材线虫的取食、繁殖和致病力密切相关,为揭示松材线虫cyp-33D3基因的功能提供了理论依据,对科学指导松材线虫病生物防治具有重要的理论和实践意义。然而,关于cyp-33D3基因对松材线虫卵壳形成和致病力的具体调控机理还不清楚,还有待于进一步深入研究。
-
通过RNA干扰技术对松材线虫cyp-33D3基因功能进行了研究,发现cyp-33D3dsRNA 干扰可以有效抑制cyp-33D3基因的表达,cyp-33D3基因干扰后降低了松材线虫的取食速度,每条线虫的产卵数量以及虫卵的孵化率都下降显著(P < 0.01)。然而,cyp-33D3基因干扰对松材线虫个体体长影响较小。cyp-33D3基因干扰减弱了松材线虫的致病能力,降低了黑松的发病率。本研究不仅为阐明松材线虫cyp-33D3基因的功能提供了数据支撑,而且为研究松材线虫其他基因的功能提供了参考。








 DownLoad:
DownLoad: