-
生活史对策理论重点关注植物繁殖过程中的资源分配过程,探讨生殖资源分配格局对特定生存环境的适应性策略。植物的生殖对策主要取决于遗传因素,但生境条件也会在一定程度上影响着植物的生殖资源分配过程。植物采取降低生物量及植物体大小以及增加生殖投资量等适应性策略来应对外界生存环境的变化[1]。有性繁殖植物的生殖成功也同样受到植物的开花量影响[2-3]。植物的生殖活动能否成功主要取决于外界各种生物环境因子和非生物环境因子的综合性作用,该过程可以利用花粉限制假说[4-5]、生境资源限制假说[6]、密度制约假说[7]、生殖个体自身大小依赖[8-9]等机制加以解释。对于风媒植物而言,花粉数量供给不足、花粉质量偏低都会影响到果实的形成。与虫媒植物相比,风媒植物具有相对更高的传粉效率。前人研究显示花粉限制作用以及生境资源限制作用,都显著地影响着风媒植物的生殖成功效率[10-11]。对于不同物种而言,花粉限制作用对坐果率的影响也是不尽相同的[12]。
对于雌花与雄花着生在不同株体上的树种来说,雌雄个体在繁殖资源投入上有着明显差别。与雄树相比,雌树由于开花、结果需要付出相对更高的生殖代价。簇毛槭是一种典型的雌雄异株物种,也是检验生殖资源分配与生殖成功限制因子的理想对象。本研究基于对簇毛槭不同性别个体的定位研究,系统地检验了生境资源限制、花粉限制、密度制约以及植株大小对生殖成功的影响。
HTML
-
实验区位于吉林省林业实验区国有林保护中心东大坡,气候类型属于温带大陆性山地气候。年平均温度为3.8℃,最热月7月的日平均温度为21.7℃,最冷月1月的日平均温度为−18.6℃,年均降水量可达695.9 mm,土壤深度在20~120 cm之间。林型为原始阔叶红松林经采伐干扰后形成的温带针阔混交林。优势树种主要包括红松(Pinus koraiensis Sieb. et Zucc.)、水曲柳(Fraxinus mandshurica Rupr.)、紫椴(Tilia amurensis Rupr.)等。在实验区内建立一块面积为21.12 hm2(660 m × 320 m)的固定监测样地。把整个样地划分成20 m × 20 m的528个连续样方,以样方为基础,系统调查全部胸径 ≥ 1 cm个体的物种名、胸高直径、树木高度、冠幅大小以及林木在样地中的相对坐标。实验样地2009年完成首次植被清查,在2014年、2019年完成两次样地复测。
-
使用由Harms提出的方法计算单个20 m × 20 m样方的地形变量[13]。测定的地形变量包括海拔高度、坡向、坡度以及凸凹度。单个样方的平均海拔利用该样方的全部顶点的海拔平均值来计算。单个样方的凸凹度用该样方的平均海拔高度值,减去与其邻接的其它样方的海拔均值表示。任取样方的3个顶点可以构成一个水平面,全部顶点能够构成4个水平面。计算样方的4个水平面与其投影面之间的夹角,将4个夹角的平均值作为其坡度值。将坡的正南向定为0°,按顺时针的方向正西向为90°,正北向为180°,正东向为270°。
于实验当年的5—8月份,在野外定位观测183株簇毛槭(包括38株雌株,145株雄株)的生殖过程。为了量化不同的生物量组分,对监测样地内所选定的簇毛槭样株,在每个样株上标记10个可以代表其平均水平的枝条。在当年的开花峰期以及果实成熟期,统计开花、结果和叶片的数量,并外推到整个样株的开花量、结实量和叶片数。收集对应的花、果实、叶片样本,在实验室烘干并称质量。投入生殖活动的生物量用当年的花及果实的干质量之和表示。投入营养生长的生物量用当年生的叶片及新生枝条的干质量之和表示。利用土壤水分测定仪测量每个簇毛槭根部对应的土壤水分状况。利用冠层分析仪测定每个簇毛槭树冠上方的叶面积指数,用来表征其生活环境的光照状况。
-
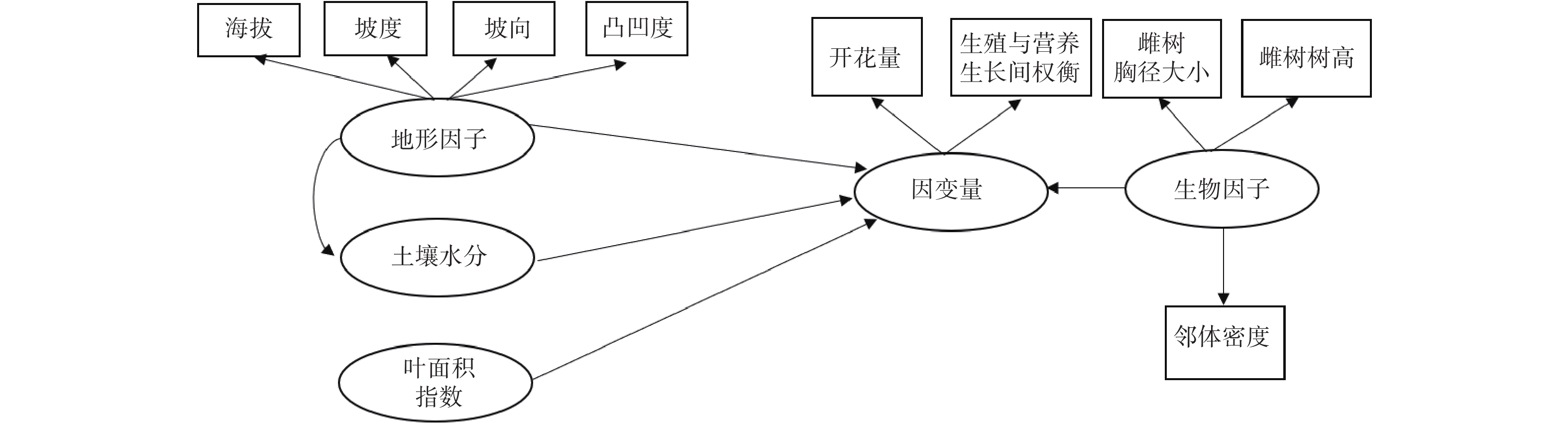
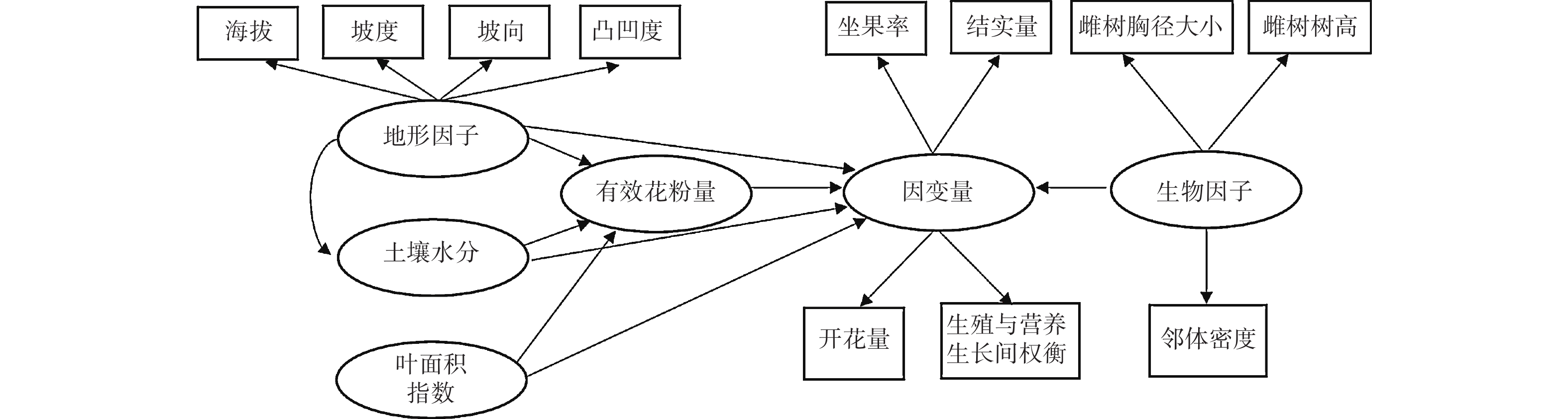
路径分析是多元回归方法的拓展,通过对自变量与响应变量间相关分解量化二者间的作用关系[14-15]。路径分析方法的组织架构图可以直观地表达不同的变量间的相互关系。由于个别繁殖数据不完整,本研究的路径分析最终使用了27个雌株、143个雄株的花、果实、营养生长数据。利用观测数据计算每个雌性植株的坐果率、开花量、结实量、生殖营养比。生殖营养比用投入到生殖生长中的资源量(花及果干质量)与投入到营养生长中的资源量(叶及新生枝干质量)之比表示。对于雄树则计算每个植株的开花量和生殖营养比。坐果率用结实量与开花量之比值来计算。利用路径分析来量化雌性植株的生殖过程的影响因素(图1)。把坐果率、开花量、结实量以及生殖营养比作为响应变量。将表征地形条件、土壤水分条件、光照条件、自身生长状况、邻体环境状况的变量作为解释性变量。
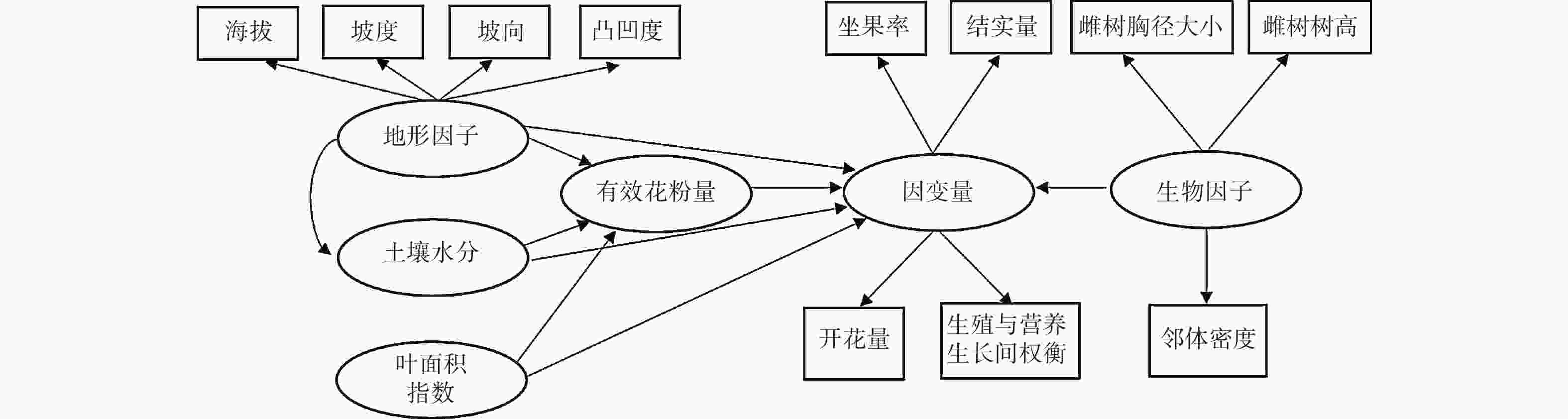
Figure 1. Path analysis diagram reflecting the effects of biotic and abiotic variables on the ratio of fruit set, fruit number, flower number and the trade-offs between reproductive and vegetative growth for female trees.
有效花粉量计算如下:
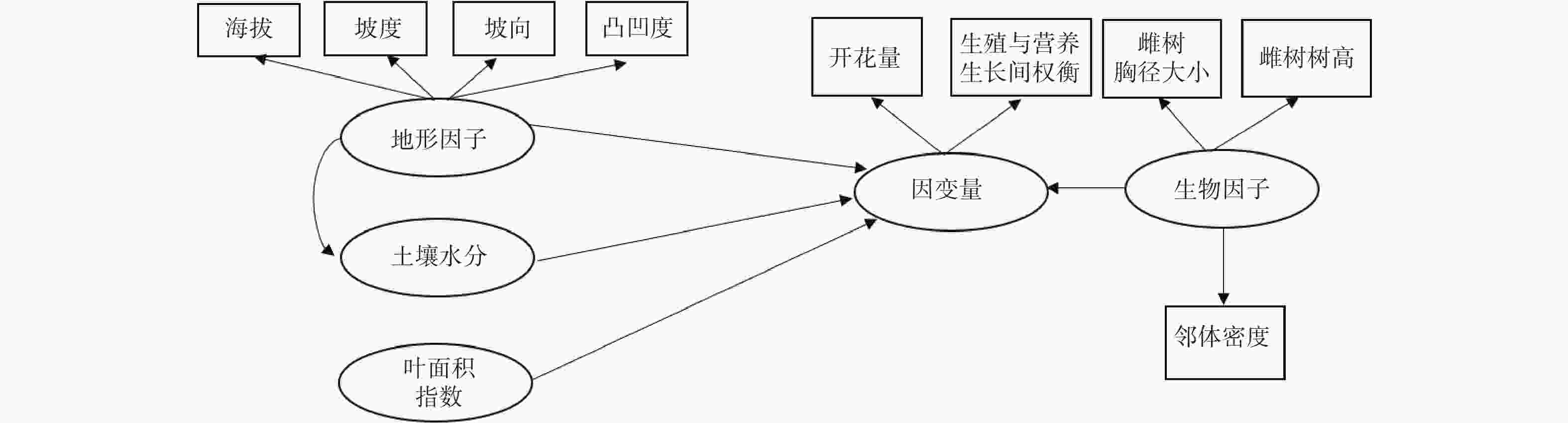
$PQ =\sum\nolimits_{i=1}^{n}\frac{{NF}_{i}}{{D}_{i}} $ 。式中,PQ表示周围相邻雄性个体可为雌性个体提供的花粉数量;NFi表示以雌性个体为中心的60 m半径范围内,雄性个体i的开花量;Di代表特定的雄性个体i与中心雌性个体的间距。利用BAL指数表示相邻林木密度状况,用以雌性个体为中心、10 m半径范围内胸径大于该雌性个体的全部相邻林木胸径的和表示[16-19]。在检验雄性个体的生殖影响因素时,依次用开花量和生殖营养比作为响应变量;采用的解释性变量与雌性个体略有不同,在分析路径中不再考虑有效花粉量(图2)。
1.1. 研究区概况
1.2. 研究方法
1.3. 数据分析
-
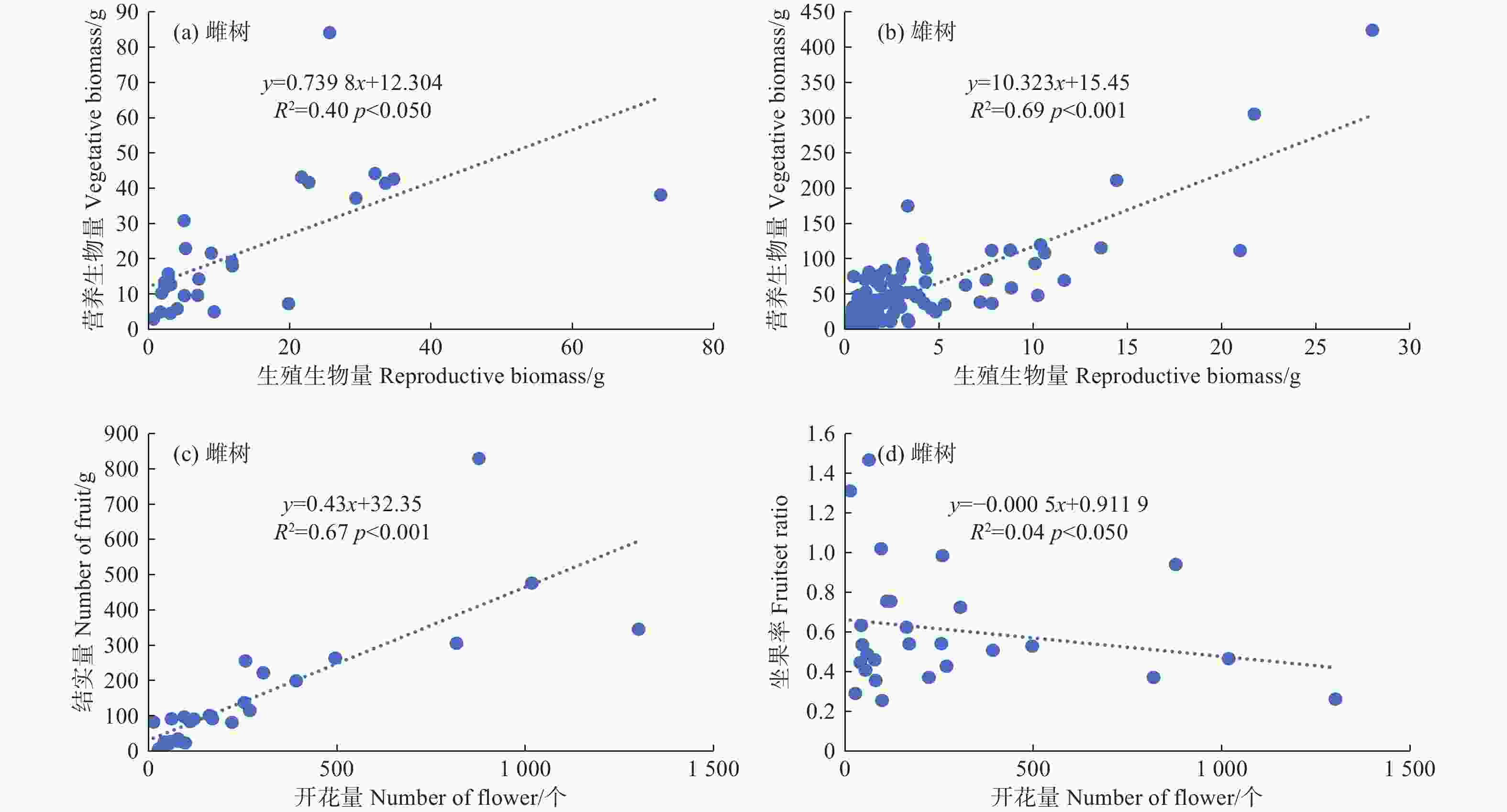
利用线性回归检验簇毛槭雌性个体的生殖权衡关系,发现生殖生长和营养生长在p < 0.05水平上呈正向的线性关系,模型解释量达到40%。这意味着当雌性个体用于生殖生长的生物量增加时,用于营养生长的生物量也会随之线性增大。雄性个体存在类似的生殖权衡现象,生殖生长与营养生长在p < 0.001水平上呈正向的线性关系,模型解释量高达69%。雌性个体的开花量与结实量之间存在显著的线性相关(R2 = 0.67,p < 0.001),植株的结实量会随着开花量的增大而增大;而雌性个体的坐果率与开花量之间的相关性并不显著(图3)。
-
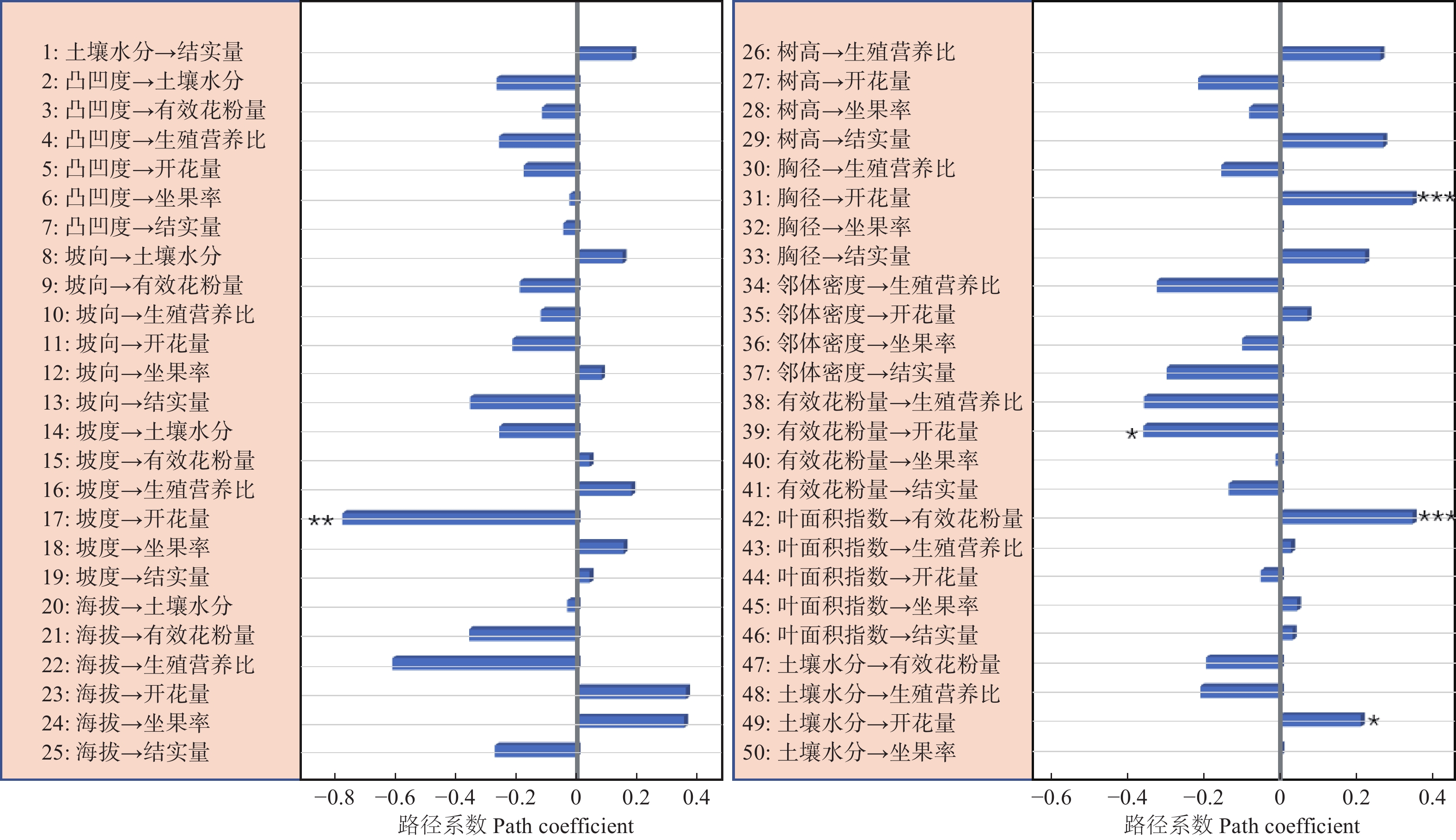
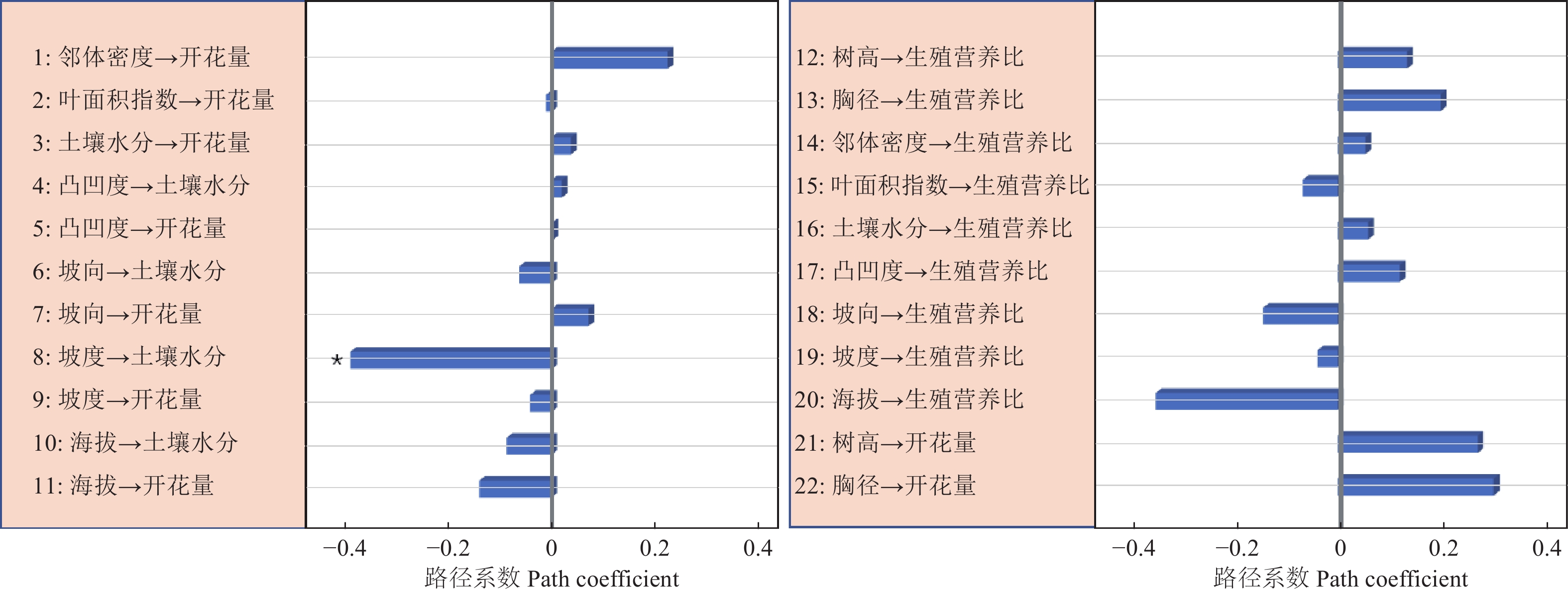
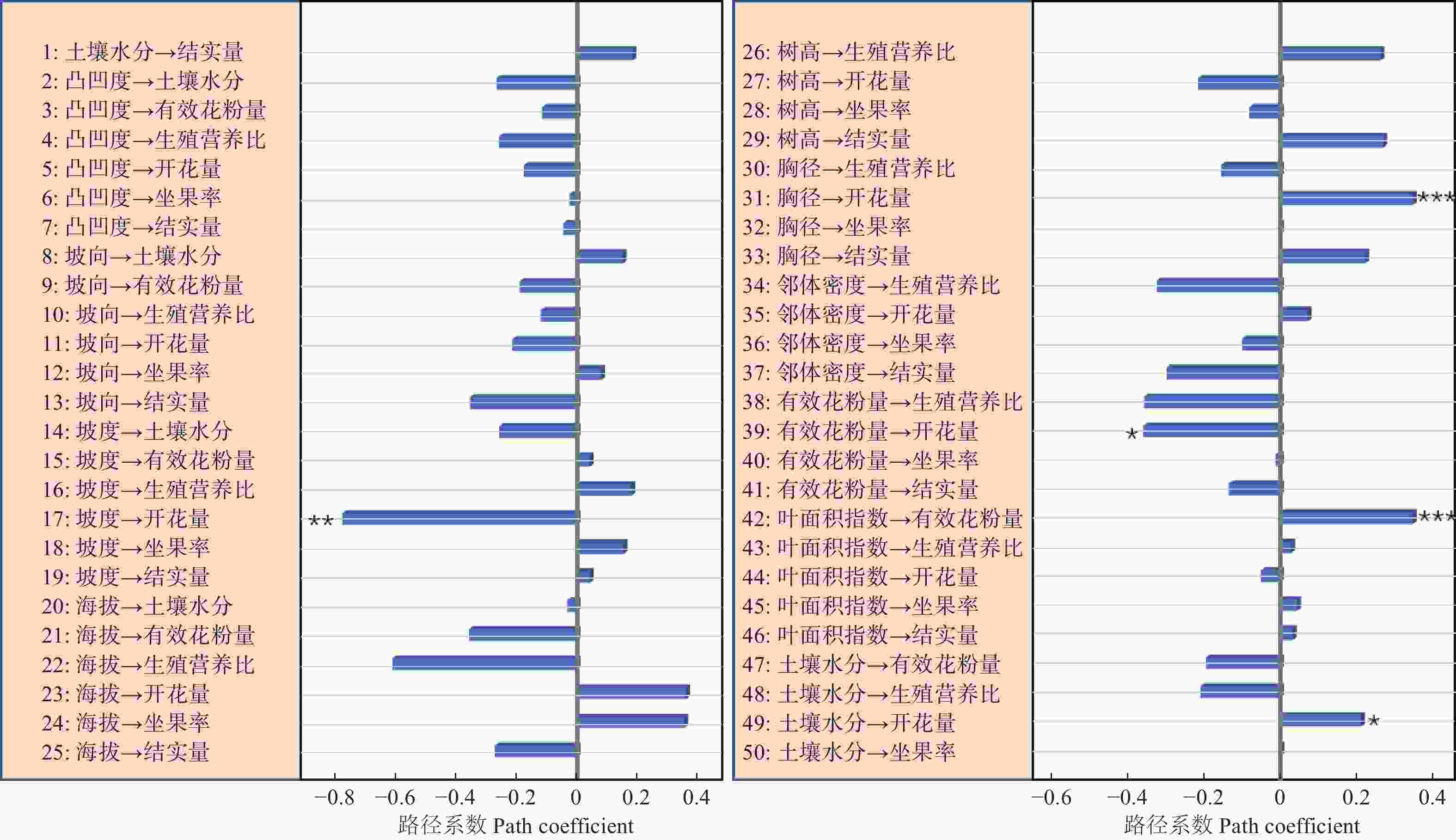
路径分析结果显示生物因子及非生物因子显著地影响雌性个体的开花量,但对坐果率、结实量和生殖营养比未表现出显著的直接作用(图4)。坡度、有效花粉量对雌株开花量具有显著的负向影响,路径系数分别为−0.78和−0.41。土壤水分、雌株胸径大小对雌株开花量具有显著的正向影响,路径系数分别为0.24和0.67。叶面积指数显著地直接作用于有效花粉量(路径系数为0.58),因而叶面积指数所反映的光照状况会间接地作用于雌性个体的开花量。路径分析显示外界环境变量、自身生长状况与雄性个体的开花量及生殖营养比之间均没有显著的作用关系(图5)。
2.1. 生殖生长与营养生长之间的关系
2.2. 生殖资源分配的影响因子分析
-
生活史对策理论认为生殖资源的分配格局与植物的生活史之间存在密切关系。大量研究显示用于生殖过程的资源量与用于营养生长或存活的资源量间存在此消彼长的关系[20-21]。本研究发现不论雌性还是雄性个体,簇毛槭生殖生长和营养生长之间都呈现出正向相关。同时检验了植株水平上的生殖分配格局,并没有发现存在生殖权衡现象。树枝进行繁殖时要投入大量资源,不可避免会降低自身的营养生长。而没有开花结实的枝条将大量资源主要用于营养生长,二者综合在一起导致无法从植株水平观测到生殖权衡现象的存在。在植株的不同发育阶段以及植株的不同构件水平上生殖权衡关系都可能是不同的,这意味着基于枝条及植株等不同水平研究生殖权衡是十分必要的。
资源限制理论发现个体的资源分配受生境中可利用性资源数量影响。当植物生存的现实环境中可利用性资源数量不足时,个体主要把自身的有效资源用于营养过程之中,以提高植物体从外界获取资源的能力[22]。本研究发现坡度大小对簇毛槭的雌性个体开花量具有显著的负向影响,土壤水分则对簇毛槭的雌性个体的开花量具有显著的正向影响。而地形因子、光照条件等生境资源状况对植株的结实量、坐果率以及生殖权衡都没有直接作用。这意味着生境资源状况与雌性个体的开花量间具有显著的作用关系,而与生殖资源分配之间不存在显著的作用关系。雄性个体的生殖分配格局也无法利用生境资源限制理论来进行解释。
花粉限制假说是用来解释植物有性生殖成功的一个重要理论。植物普遍表现出可以成功授粉坐果的花的比例较低[23-24]。因而,植物坐果率大小主要是由花粉限制决定的。本研究显示相邻雄性个体的有效花粉量与雌株开花量之间具有显著的作用关系,但与生殖分配格局间并不存在作用关系。因此,花粉限制假说无法很好地阐释生殖与营养间的资源分配机制。
密度大小已经被证实是制约个体资源分配的关键变量,当密度增大时会对应地降低植株的生殖产量[7]。前人对华北驼绒藜 (Ceratoides arborescens (Losinsk.) Tsien et C.G.Ma)进行了相关的研究,发现用于生殖过程的资源量与群落盖度以及种群自身的相对盖度之间具有显著的相关性。并且生殖产量与群落的覆盖度之间具有显著的负向关系,与自身种群密度之间具有显著的正相关关系[25]。此外对红葱 (Eleutherine bulbosa (Mill.) Urb.)的相关研究显示,当密度增大时投入到地上部分、叶片及叶鞘上的生物量比例会增大,而投入到地下部分以及鳞茎上的比例则对应减少。投入到营养器官的资源量比例随着密度的增大而增大,并会对应降低无性繁殖器官的资源分配量[26]。
生殖分配格局与自身状况具有显著的相关性,用于生殖的资源量会随着植株年龄的增大呈线性增加[8]。与此不同的是,川鄂连蕊茶(Camellia rosthorniana Hand.-Mazz.)的生殖资源量与个体大小之间呈现出抛物线关系[27];对黄帚橐吾 (Ligularia virgaurea (Maxim.) Mattf. ex Rehd. & Kobuski)的研究显示,生殖资源分配与其自身大小有关,投入到生殖过程的资源量与植株大小呈负向关系[28];类似的研究显示,投入到生殖过程的资源量随着个体大小的增大呈现由低到高再到低的规律,且不同的生活环境下生殖分配量的差异很大[25]。本研究表明,簇毛槭雌性个体的胸径大小对其开花量的多少具有显著的正向影响,但自身大小对雌性及雄性个体的生殖分配过程都没有显著的影响。
-
本研究结果表明,簇毛槭的营养生长与生殖生长间存在着显著的权衡关系,环境因子及花粉限制等因素对生殖成功的影响很小。







 DownLoad:
DownLoad: