-
腥臭卫矛(Euonymus sanguineus Loes. var. paedidus L. M. Wang)是卫矛属(Euonymus L.)植物石枣子(E. sanguineus Loes.)的变种,落叶灌木,秋季叶色变红,为彩色叶树种。二歧聚伞花序,花基数4,花瓣卵形,淡黄色;花期4月底至6月,开花时植株周围弥漫着强烈的腥臭味,是迄今发现的唯一具刺激性气味的卫矛属植物[1-2],有些人近距离吸入会有头痛、恶心等不良反应,花凋谢后刺激性气味随之消失。卫矛属植物大多用于杀虫消毒、治疗跌打损伤、活血止血及抗肿瘤等作用。目前,已对卫矛(E. alatus (Thunb.) Sieb.)[3-4]、扶芳藤(E. fortune (Turcz.) Hand.-Mazz.)[5]、冬青卫矛(E. japonicas Thunb.)[6]、白杜(E. maackii Rupr)[7]、刺果卫矛(E. acanthocarpus Franch.)[8-9]、疏花卫矛(E. laxiflorus Champ. ex Benth.)[10]、黄心卫矛(E. macropterus Rupr.)[11]、陕西卫矛(E. schensianus Maxim.)[12]等多个树种的化学成分进行了分析,主要成分有生物碱、萜类、黄酮类、甾体和强心苷等,如卫矛碱、异卫矛碱、雷公藤碱、卫矛羰碱、新卫矛羰碱、槲皮素、香橙素、儿茶素、无羁萜、熊果醇、β-谷甾醇、卫矛酮、草酰乙酸钠等,这些成分并未使植株在生命周期中产生不良气味。
植物器官或组织随着结构的分化和成熟,所含挥发性成分的种类和相对含量会产生不同程度的差异[13-16]。那么,使腥臭卫矛在开花时呈现刺激性气味的主要挥发性成分是什么?由花的哪个结构释放?这些问题未见研究报道,制约了对腥臭卫矛的认知与应用。
顶空固相微萃取-气相色谱-质谱联用技术(SPME-GC-MS)是研究挥发性成分的有效手段[17-19],为了探明与腥臭卫矛开花时所释放刺激性气味相关的化合物和结构来源,利用SPME-GC-MS法,对腥臭卫矛花序中不同发育时期的花(苞)以及花绽开时的花瓣和花盘挥发性成分进行了分析鉴定,为腥臭卫矛刺激性气味的产生提供科学参考,为卫矛属植物的分类、演化与应用提供化学依据。
HTML
-
供试材料为新鲜腥臭卫矛二歧聚伞花序中不同发育时期的小花(苞),于2018年4—5月采于山西农业大学植物园。小花苞形成(V1),花苞膨大呈绿色(V2),花苞膨大顶端露出部分黄色花瓣(V3),花瓣完全露出花萼但聚拢未展开(V4),小花完全展开(V5),小花(苞)各时期V1、V2、V3、V4、V5宽度(最宽处)分别为1.43±0.24、1.88±0.11、2.84±0.06、3.16±0.10、8.25±0.13 mm。解剖处于V5期小花的花瓣(Y1)与花盘(Y2)两部分亦作为试验材料。
50/30 μm DVB/CAR/PDMS三相萃取头,美国Supelco公司;Triplus RSH型多功能自动进样器,美国Thermo Scientific公司;OM-5MS(30 m × 0.25 mm × 0.25 μm)色谱柱,美国Omni Gene LLC公司;Trace 1300 ISQ气相色谱质谱联用仪,美国Thermo Scientific公司。
-
根据Uekane等[20]的方法并做相应修改,即取供试材料各0.5 g置入5 mL顶空瓶中,在50℃下萃取30 min后,萃取头于气相色谱进样口250℃解吸5 min,进行GC-MS分析。
-
载气为氦气,进样口温度250℃,柱温采用程序升温,40℃初始温度保持3 min,以4℃·min−1升至160℃,再以20℃·min−1升至240℃,保持5 min不分流进样。
-
离子源为EI源;离子源温度为280℃;质谱传输线280℃;电子能量为70 eV;质核比扫描范围30~500 amu。
-
GC-MS分析所得总离子流色谱图(图1),不同阶段分离出的化合物,试验出报告时根据含量多少取前100种进行后续分析。各组分经NIST(MS Search 2.0)标准质谱库检索比对,并参考已发表文献[21]进行初步确认,再与Adams[22]所述各化合物出峰顺序进行核对,按峰面积归一化法确定成分含量。运用Excel 2016软件对所得的成分进行统计分析,并用XLSTAT2018软件进行主成分分析(PCA),利用SPSS21.0软件进行多重比较(MC)和聚类分析(HCA)。
2.1. 腥臭卫矛花SPME条件
2.2. 腥臭卫矛花GC-MS条件
2.2.1. 色谱条件
2.2.2. 质谱条件
2.3. 数据分析
-
采用SPME-GC-MS法对腥臭卫矛花的挥发性成分进行分析,共鉴定出58个化合物(表1),总含量范围从96.51%(V2期)到98.56%(V5期),相对含量较高的化合物包括芳樟醇(0.15%~70.79%)、叶醇(0.41%~65.51%)、苯乙腈(0.04%~62.20%)、2-乙烯基-1,1-二甲基-3-亚甲基环己烷(0.02%~32.99%)、(E)-β-罗勒烯(0.25%~21.26%)、α-蒎烯(0.02%~20.41%)、D-柠檬烯(1.01%~11.74%)、水杨酸甲酯(0.14%~6.14%)。
序号
No.保留时间
Retention time/min化合物
Components相对质量分数 Contents/% V1 V2 V3 V4 V5 Y1 Y2 1 2.48 3-甲基呋喃 3-Methylfuran 1.15 ± 0.15 a 0.59 ± 0.28 b 0.31 ± 0.04 bcd 0.17 ± 0.03 cd 0.10 ± 0.00 d 0.08 ± 0.05 d 0.45 ± 0.04 bc 2 3.34 1-戊烯-3-醇 1-Penten-3-ol 0.83 ± 0.02 a 0.74 ± 0.12 a 0.48 ± 0.03 b 0.37 ± 0.10 b 0.18 ± 0.03 c 0.03 ± 0.02 c 0.12 ± 0.01 c 3 4.47 异戊醇 Isoamyl alcohol 0.41 ± 0.01 a 0.32 ± 0.05 b 0.22 ± 0.03 c 0.12 ± 0.02 d 0.07 ± 0.03 de 0.01 ± 0.01 e 0.12 ± 0.05 d 4 5.38 (Z)-2-戊烯醇 (Z)-2-Pentenol 0.27 ± 0.02 a 0.16 ± 0.02 b 0.10 ± 0.01 c 0.07 ± 0.02 cd 0.04 ± 0.01 de 0.02 ± 0.01 e 0.06 ± 0.00 cd 5 6.01 己醛 Hexanal 0.24 ± 0.04 a 0.21 ± 0.07 a 0.10 ± 0.02 b 0.08 ± 0.03 bc 0.04 ± 0.02 bc 0.01 ± 0.00 c 0.06 ± 0.01 bc 6 7.87 叶醇Leaf alcohol 65.51 ± 0.40 a 62.26 ± 0.45 a 26.56 ± 4.09 b 17.14 ± 3.04 c 9.86 ± 1.64 d 0.41 ± 0.11 e 7.37 ± 0.33 d 7 8.25 (Z)- 3-己烯醇 (Z)- 3-Hexenol 2.25 ± 0.40 a 1.11 ± 0.24 b 0.31 ± 0.05 c 0.17 ± 0.01 c 0.04 ± 0.03 c - 0.24 ± 0.04 c 8 8.38 顺-2-己烯醇 (Z)-2-hexenol 0.01 ± 0.01 b - 0.02 ± 0.03 a - 0.03 ± 0.02 a - 0.08 ± 0.03 a 9 9.12 2-甲基-4-戊烯醇
2-methyl-4-Pentenol0.09 ± 0.02 a 0.05 ± 0.01 b - - - 0.01 ± 0.00 c 0.04 ± 0.00 b 10 10.11 甲酸叶醇酯
(Z)-3-Hexenyl formate- - - - 0.04 ± 0.00 b 0.07 ± 0.02 a 0.04 ± 0.01 b 11 10.20 三环萜 Tricyclene 0.04 ± 0.02 a 0.04 ± 0.00 a - 0.01 ± 0.01 b - - - 12 10.58 α-蒎烯 α-Pinene 0.02 ± 0.02 d 0.07 ± 0.01 d 0.39 ± 0.24 d 2.62 ± 0.97 cd 10.90 ± 1.23 b 20.41 ± 4.57 a 6.28 ± 1.87 c 13 10.67 (Z)-3-己烯酸甲酯
(Z)-3-Hexenoic acid methyl ester0.13 ± 0.01 a 0.09 ± 0.01 b - - - - - 14 11.10 莰烯 Camphene - - 0.03 ± 0.01 c 0.11 ± 0.03 c 0.40 ± 0.05 b 0.79 ± 0.21 a 0.31 ± 0.11 b 15 11.79 苯甲醛 Benzaldehyde - - - - - - 0.08 ± 0.00 a 16 12.18 桧烯 Sabinen - - - - - - 0.26 ± 0.08 a 17 12.20 β-蒎烯 β-Piβ-Pinene - - - 0.10 ± 0.03 c 0.45 ± 0.06 b 0.86 ± 0.27 a - 18 12.85 β-月桂烯 β-Myrcene 0.08 ± 0.01 d 0.13 ± 0.04 cd 0.14 ± 0.02 cd 0.20 ± 0.03 c 0.41 ± 0.03 b 0.52 ± 0.04 a 0.10 ± 0.03 d 19 13.51 乙酸叶醇酯 Leaf acetate 2.83 ± 0.34 a 2.50 ± 0.26 a 0.93 ± 0.08 b 1.01 ± 0.54 b 0.60 ± 0.23 bc 0.02 ± 0.01 c 0.08 ± 0.03 c 20 14.23 D-柠檬烯 D-Limonene 5.21 ± 1.68 b 11.74 ± 0.78 a 1.36 ± 0.40 c 1.01 ± 0.33 c 2.00 ± 0.53 c 1.80 ± 0.07 c 1.25 ± 0.36 c 21 14.36 桉叶油醇 Eucalyptol - - 0.08 ± 0.03 b 0.61 ± 0.09 a - - 0.47 ± 0.10 a 22 14.64 β-罗勒烯 β-Ocimene 0.05 ± 0.01 c 0.05 ± 0.01 c 0.71 ± 0.11 a 0.32 ± 0.03 b 0.30 ± 0.02 b 0.23 ± 0.00 b 0.22 ± 0.03 b 23 15.02 (E)-β-罗勒烯 (E)-β-Ocimene 0.71 ± 0.31 c 1.00 ± 0.13 c 21.26 ± 3.05 a 5.96 ± 0.92 b 1.28 ± 0.15 c 0.25 ± 0.01 c 2.70 ± 0.92 c 24 15.40 γ-松油烯 γ-Terpinene 0.02 ± 0.01 cd 0.01 ± 0.01 d 0.12 ± 0.02 bc 0.04 ± 0.00 cd 0.18 ± 0.05 ab 0.08 ± 0.02 bcd 0.25 ± 0.11 a 25 16.04 (Z)-氧化芳樟醇
(Z)-Linalool oxide0.03 ± 0.01 c 0.05 ± 0.01 c 0.04 ± 0.01 c 0.09 ± 0.02 b 0.12 ± 0.01 b 0.16 ± 0.04 a 0.03 ± 0.01 c 26 16.62 (E)-氧化芳樟醇
(E)-Linalool oxide- - 0.09 ± 0.01 b 0.23 ± 0.01 a 0.23 ± 0.01 a 0.24 ± 0.05 a 0.25 ± 0.01 a 27 16.76 苯甲酸甲酯
Methyl benzoate- - - 0.04 ± 0.02 b 0.06 ± 0.01 b 0.16 ± 0.04 a - 28 16.85 1,3,7-三甲基-2,6-二烯乙酸酯
Acetic acid 1,3,7-trimethylocta-2,6-dienyl ester0.20 ± 0.04 c 0.20 ± 0.02 c 0.84 ± 0.08 a 0.40 ± 0.09 b 0.13 ± 0.05 c - 0.23 ± 0.06 c 29 17.06 芳樟醇 Linalool 0.16 ± 0.02 d 0.15 ± 0.04 d 5.17 ± 0.72 d 30.17 ± 2.52 c 51.21 ± 2.89 b 70.79 ± 5.09 a 3.08 ± 0.21 d 30 17.18 壬醛 Nonanal 0.42 ± 0.14 a 0.55 ± 0.25 a 0.19 ± 0.03 a 0.58 ± 0.31 a 0.39 ± 0.18 a - 0.14 ± 0.08 a 31 17.24 二氢芳樟醇 Dihydrolinalool - - 0.07 ± 0.02 b 0.02 ± 0.00 c - 0.21 ± 0.01 a - 32 17.63 2-乙烯基-1,1-二甲基-3-
亚甲基环己烷
2-ethenyl-1,1-dimethyl-3-methylene-Cyclohexane4.65 ± 1.20 d 5.30 ± 1.38 d 32.99 ± 1.18 a 19.69 ± 1.84 b 8.08 ± 0.94 cd 0.02 ± 0.01 e 10.89 ± 2.30 c 33 18.10 波斯菊萜 Cosmene - - 0.24 ± 0.04 a 0.07 ± 0.01 b 0.03 ± 0.00 b 0.02 ± 0.00 b 0.03 ± 0.01 b 34 18.38 苯乙腈 Phenylacetonitrile 0.66 ± 0.46 c 0.04 ± 0.01 c 1.65 ± 0.66 c 15.00 ± 2.40 b 10.30 ± 1.26 b - 62.20 ± 6.16 a 35 18.54 γ-焦烯 γ-Pyronene - - 0.17 ± 0.09 a - - 0.04 ± 0.01 b - 36 19.47 2-莰醇 2-Borneol - - - 0.03 ± 0.01 c 0.17 ± 0.04 b 0.45 ± 0.04 a 0.01 ± 0.00 c 37 19.76 环氧芳樟醇 Epoxylinalol - 0.02 ± 0.01 c 0.08 ± 0.01 a 0.05 ± 0.01 b 0.02 ± 0.01 c 0.01 ± 0.00 c 0.03 ± 0.01 c 38 20.21 丁酸叶醇酯 Leaf butyrate 0.71 ± 0.05 a 0.55 ± 0.10 b 0.16 ± 0.00 c 0.09 ± 0.04 c 0.03 ± 0.00 d - 0.04 ± 0.01 d 39 20.42 水杨酸甲酯 Methyl salicylate 5.79 ± 1.14 a 6.14 ± 1.59 a 0.95 ± 0.24 b 0.31 ± 0.07 b 0.14 ± 0.04 b 0.40 ± 0.02 b 0.39 ± 0.09 b 40 20.89 癸醛 Decanal 0.10 ± 0.02 ab 0.14 ± 0.03 ab 0.10 ± 0.02 ab 0.20 ± 0.11 a 0.04 ± 0.01 b 0.02 ± 0.00 b 0.04 ± 0.00 b 41 21.83 戊酸叶醇酯 Leaf pentanoate 0.08 ± 0.00 ab 0.12 ± 0.02 ab 0.14 ± 0.06 ab 0.17 ± 0.10 a 0.04 ± 0.02 b - 0.02 ± 0.01 b 42 21.98 异戊酸叶醇酯
(Z)-3-Hexenyl isovalerate0.15 ± 0.00 a - 0.07 ± 0.01 b 0.04 ± 0.02 b 0.02 ± 0.00 b - 0.02 ± 0.00 b 43 23.84 吲哚 Indole 0.04 ± 0.01 b 0.07 ± 0.04 b 0.05 ± 0.01 b 0.26 ± 0.04 a 0.23 ± 0.04 a 0.14 ± 0.13 a 0.07 ± 0.01 b 44 24.17 正十三烷 Tridecane 0.04 ± 0.02 a 0.04 ± 0.01 a 0.03 ± 0.01 a 0.02 ± 0.00 a 0.01 ± 0.00 a 0.01 ± 0.00 a - 45 25.93 2-甲基丙烯酸-2.2 -二甲基-1-(2-羟基-1-甲基)丙烯酯
2-methyl-Propanoic acid- 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl ester- - 0.08 ± 0.04 a 0.01 ± 0.00 b 0.02 ± 0.01 b 0.01 ± 0.00 b - 46 26.34 2,5-二甲基正己烷-2,5-
二甲羟基过氧化物
2,5-Dimethylhexane-2,5-dihydroperoxide0.04 ± 0.03 b 0.06 ± 0.05 b 0.19 ± 0.11 a 0.03 ± 0.01 b 0.02 ± 0.01 b 0.03 ± 0.02 b 0.02 ± 0.02 b 47 27.46 (Z)-茉莉酮 (Z)-Jasmone 0.15 ± 0.03 b 0.10 ± 0.02 c 0.09 ± 0.02 c 0.05 ± 0.00 cd 0.03 ± 0.00 d 0.03 ± 0.01 d 0.25 ± 0.01 a 48 28.00 四甲基癸炔二醇
Tetramethyl decynediol0.06 ± 0.01 b 0.13 ± 0.03 a 0.07 ± 0.00 b 0.05 ± 0.01 bc 0.02 ± 0.00 cd - 0.01 ± 0.00 d 49 28.16 异丁香烯 Isocaryophyllene 0.09 ± 0.02 a 0.05 ± 0.00 bc 0.07 ± 0.01 ab 0.04 ± 0.01 c 0.01 ± 0.01 d - - 50 28.65 α-香柑油烯 α-Bergamotene 0.07 ± 0.01 b 0.19 ± 0.06 a 0.14 ± 0.01 ab 0.08 ± 0.01 b 0.07 ± 0.03 b - 0.02 ± 0.01 c 51 29.28 (E)-β-金合欢烯
(E)-β-Famesene0.04 ± 0.03 a 0.06 ± 0.04 a 0.06 ± 0.00 a 0.02 ± 0.00 a 0.01 ± 0.00 a - 0.01 ± 0.01 a 52 30.08 大根香叶烯 D-Germacren 0.07 ± 0.03 a 0.05 ± 0.01 a 0.01 ± 0.01 b - - - - 53 30.45 (Z,E)-α-金合欢烯
(Z,E)-α-Farnesene0.09 ± 0.01 a 0.04 ± 0.00 b 0.03 ± 0.01 b - - - - 54 30.86 α-金合欢烯 α-Farnesene 3.23 ± 0.10 a 1.02 ± 0.05 b 0.18 ± 0.07 c 0.03 ± 0.00 d 0.01 ± 0.01 d - 0.05 ± 0.02 cd 55 31.36 δ-杜松烯 δ-Cadinene 0.05 ± 0.00 a 0.02 ± 0.00 b - - - - - 56 32.71 (Z)-苯甲酸叶醇酯
(Z)-3-Hexenyl benzoate0.06 ± 0.01 cd 0.10 ± 0.02 c 0.21 ± 0.03 b 0.26 ± 0.04 a 0.08 ± 0.01 c 0.02 ± 0.00 d 0.02 ± 0.01 d 57 33.47 2-甲基丙酸-1-叔丁基-2-甲基-1,3-丙烷二基酯
2-methyl propanoic acid- 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester0.10 ± 0.03 b 0.07 ± 0.01 b 0.36 ± 0.19 a 0.04 ± 0.01 b 0.04 ± 0.01 b 0.05 ± 0.03 b 0.02 ± 0.00 b 58 36.87 邻苯二甲酸二异丁酯
Diisobutyl phthalate0.15 ± 0.02 ab 0.18 ± 0.04 ab 0.09 ± 0.01 ab 0.23 ± 0.18 a 0.08 ± 0.02 ab 0.03 ± 0.02 ab 0.03 ± 0.01 b 总计 Total identified /% 97.08 ± 0.18 96.51 ± 0.99 97.57 ± 0.15 98.41 ± 0.16 98.56 ± 0.17 98.44 ± 0.18 98.48 ± 0.18 化合物组成
Grouped compounds/%烃类 Hydrocarbons 14.47 ± 0.77 d 19.77 ± 2.37 cd 57.94 ± 5.51 a 30.35 ± 0.92 b 24.13 ± 2.12 bc 25.04 ± 7.33 bc 22.37 ± 8.29 bcd 醇类 Alcohols 69.62 ± 0.87 ab 65.03 ± 0.60 bc 33.42 ± 5.03 e 49.78 ± 1.09 d 62.19 ± 1.27 c 72.36 ± 7.42 a 11.93 ± 0.02 f 含氮化合物 Nitrogenous 0.63 ± 0.53 c 0.09 ± 0.03 c 1.52 ± 0.71 c 15.88 ± 2.87 b 10.14 ± 1.45 b 0.14 ± 0.12 c 62.27 ± 9.23 a 脂类 Lipids 10.23 ± 1.45 a 10.00 ± 1.90 a 3.73 ± 0.03 b 3.77 ± 0.87 bc 1.26 ± 0.38 c 0.76 ± 0.13 c 0.87 ± 0.18 c 呋喃类 Furans 1.15 ± 0.15 a 0.59 ± 0.28 b 0.31 ± 0.04 bcd 0.27 ± 0.03 cd 0.10 ± 0.00 d 0.08 ± 0.05 d 0.45 ± 0.04 bc 醛类 Aldehydes 0.76 ± 0.20 ab 0.90 ± 0.30 a 0.38 ± 0.07 bc 0.52 ± 0.30 ab 0.47 ± 0.25 ab 0.03 ± 0.01 c 0.33 ± 0.14 b 酮类 Ketones 0.15 ± 0.03 b 0.10 ± 0.03 c 0.09 ± 0.02 c 0.05 ± 0.01 cd 0.03 ± 0.01 d 0.03 ± 001 d 0.26 ± 0.02 a 注:“-”表示未检出;同行不同小写字母表示不同发育时期间差异显著(P < 0.05)。
Notes: “-” indicated as not contain. Different letters in the same line indicated significant in different development stages at P < 0.05.Table 1. Relative content of essential oil compositions in different development stages and parts of E. sanguineus var. paedidus flowers
不同发育时期的腥臭卫矛花挥发性成分差异较大,V1期共鉴定出44种成分,其含量合计达97.08%,主要成分为叶醇(65.51%)、水杨酸甲酯(5.79%)、D-柠檬烯(5.21%),占全部成分的76.51%;V2期共鉴定出43种成分,其含量合计达96.51%,主要成分为叶醇(62.26%)、D-柠檬烯(11.74%)、水杨酸甲酯(6.14%)、2-乙烯基-1,1-二甲基-3-亚甲基环己烷(5.30%),占全部成分的85.44%;V3期共鉴定出48种成分,其含量合计达97.57%,主要成分为2-乙烯基-1,1-二甲基-3-亚甲基环己烷(32.99%)、叶醇(26.56%)、(E)-β-罗勒烯(21.26%)、芳樟醇(5.17%),占全部成分的85.98%;V4期共鉴定出48种成分,其含量合计达98.41%,主要成分为芳樟醇(30.17%)、2-乙烯基-1,1-二甲基-3-亚甲基环己烷(19.69%)、叶醇(17.14%)、苯乙腈(15.00%)、(E)-β-罗勒烯(5.96%),占全部成分的87.96%;V5期共鉴定出47种成分,其含量合计达98.56%,主要成分为芳樟醇(51.21%)、α-蒎烯(10.90%)、苯乙腈(10.30%)、叶醇(9.86%)、2-乙烯基-1,1-二甲基-3-亚甲基环己烷(8.08%),占全部成分的90.35%。由花苞分化到开花,叶醇显著减少,芳樟醇显著增多,苯乙腈激增。
花瓣与花盘的挥发性成分也存在较大差异,花瓣(Y1)共鉴定出37种成分,其含量合计达98.44%,主要为芳樟醇(70.79%)和α-蒎烯(20.41%),占全部成分的91.20%;花盘(Y2)共鉴定出46种成分,其含量合计达98.48%,主要为苯乙腈(62.20%)、2-乙烯基-1,1-二甲基-3-亚甲基环己烷(10.89%)、叶醇(7.37%)、α-蒎烯(6.28%),占全部成分的86.74%。
-
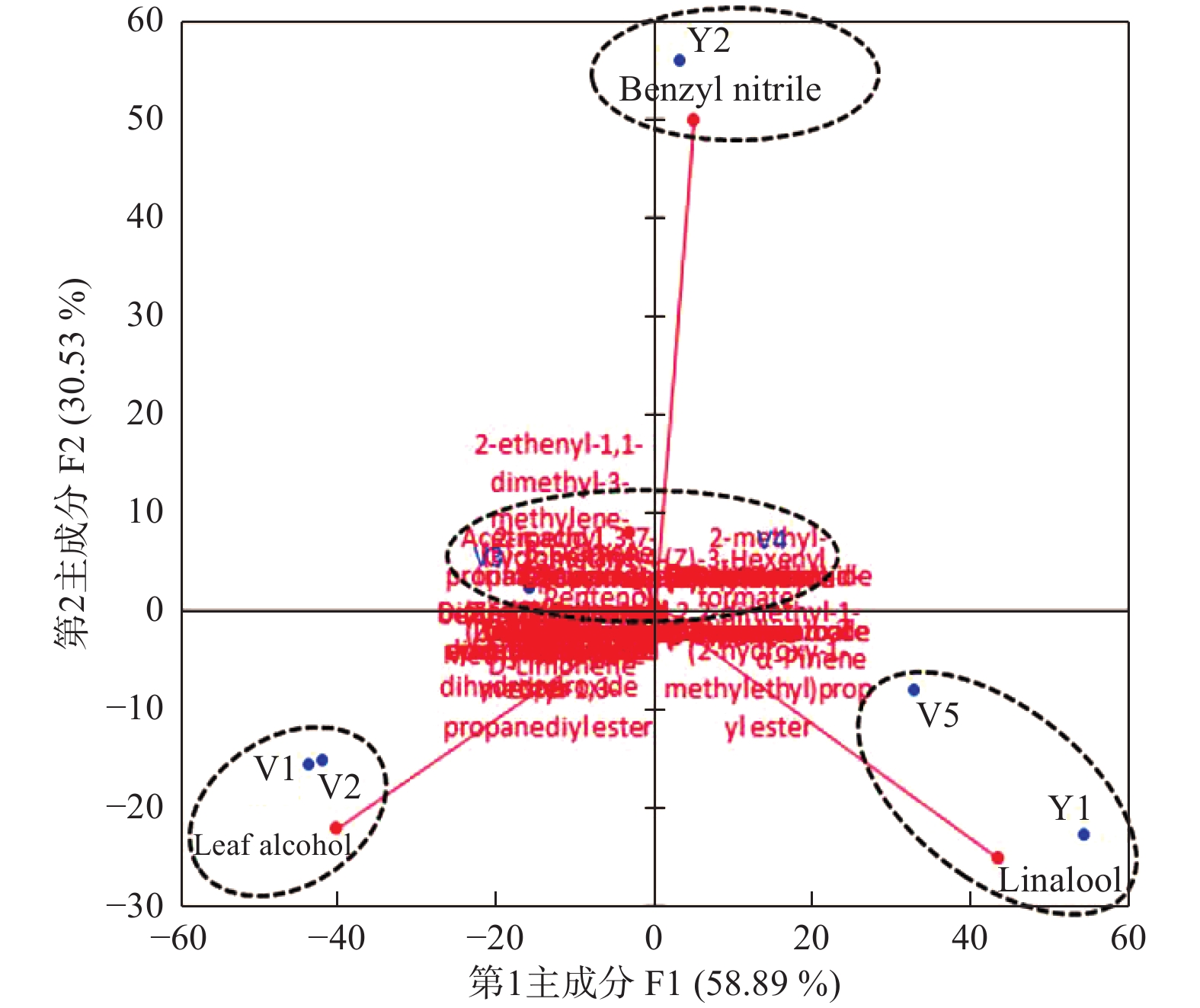
以58种腥臭卫矛花挥发性成分均值构成7 × 58的矩阵,运用XLSTAT 2018软件进行PCA分析,结果(图2)表明:第1主成分(F1)方差贡献率为58.89%,第2主成分(F2)方差贡献率为30.53%,累计贡献率达89.42%,说明前2个主成分可代表样品主要挥发性成分的组成。因此,提取前2个主成分进行腥臭卫矛花的特征挥发性成分分析。第1主成分中异戊醇、己醛、1-戊烯-3-醇、芳樟醇、叶醇、α-蒎烯和2-莰烯的贡献率最大,对应的特征向量均大于0.80;第2主成分中苯乙腈、苯甲醛、桧烯和顺-2-己烯醇贡献最大,对应的特征向量分别为0.923、0.866、0.866、0.806。

Figure 2. Biplot obtained by PCA of chemical constituents of E. sanguineus Loes. var. paedidus L. M. Wang flowers
腥臭卫矛花发育的5个时期与开花期不同部位在PCA双标图中被聚成4大类(化学型),其中,V1和V2期聚为1类,属于叶醇型;V3与V4期聚为1类,此类成分复杂,化学型不明显;V5期和Y1(花瓣)聚为1类,为芳樟醇型;Y2(花盘)独自为1类,属于苯乙腈型。聚类分析结果(图3)与主成分分析结果完全一致,亦可将其分为4大类。可见叶醇、芳樟醇和苯乙腈3种成分在腥臭卫矛花发育的不同时期及部位中相对含量差异显著,可以用于区分花发育的时期及花瓣和花盘。
-
腥臭卫矛开花期的刺激性气味为强烈的鱼腥草气味,起初推测该气味主要来源于鱼腥草素(癸酰乙醛),在腥臭卫矛花中并未检出鱼腥草素,腥臭卫矛开花期的刺激性气味与鱼腥草素无关。
在腥臭卫矛开花时含量激增的成分可能与刺激性气味有关,主要是苯乙腈。苯乙腈俗名氰化苄,具刺激性,吸入后出现头痛头晕、恶心、呕吐、倦睡、上呼吸道刺激、神志丧失等,可引起死亡。苯乙腈在V5期的花瓣中未检出,在花盘中相对质量分数高达62.20%,说明苯乙腈主要由花盘中释放。
虽然V5期存在大量香气成分,如芳樟醇(51.21%)、α-蒎烯(10.90%)、叶醇(9.86%)、D-柠檬烯(2.00%)、(E)-β-罗勒烯(1.28%)等,但是花盘释放高含量的苯乙腈掩盖了美好的气味,使植株呈现刺激性气味。另外,V5期的花瓣和花盘中分别含有微量的2-莰醇(0.45%和0.01%),也可能是刺激性气味的来源成分。2-莰醇,吸收后有微毒,可能引起过敏反应,接触后可引起头痛、恶心、呕吐及惊厥[23]。
3.1. 腥臭卫矛花挥发性成分分析
3.2. 腥臭卫矛花挥发性化合成分PCA和HCA分析
3.3. 腥臭卫矛开花期刺激性气味分析
-
本研究利用SPME-GC-MS技术,对腥臭卫矛花序中不同发育时期的花(苞)及花绽开时的花瓣和花盘中的挥发性成分进行了分析鉴定,从中鉴定出了绝大多数(96.51%~98.56%)成分。研究表明,腥臭卫矛花的挥发性成分主要为芳樟醇、叶醇、苯乙腈、2-乙烯基-1,1-二甲基-3-亚甲基环己烷、(E)-β-罗勒烯、α-蒎烯、D-柠檬烯和水杨酸甲酯,且腥臭卫矛花不同发育时期或开花期花瓣和花盘中的挥发性成分差异较大。从腥臭卫矛开花期(V5)鉴定出了47种成分,未检出53种成分,其中,未检出成分含量占比极少,仅1.13%,未检出成分种类庞杂且占比极少,对腥臭卫矛花的风味影响不大。腥臭卫矛开花时激增的刺激性成分主要为苯乙腈,且仅存在于花盘中,占总成分的62.20%。
苯乙腈属芳香性烃类物质,在玛咖(Lepidium meyenii Walp.)根[24]、日本枇杷(Eriobotrya japonica (Thunb.) Lindl.)花[25]以及某些柑橘属植物品种(Citrus unshiu Blanco ‘Chahara’、C. unshiu Blanco ‘Okitsu’、C. unshiu Blanco ‘Zorica’)的蜜腺[21]等植物器官中均有发现,但并未使植物本身产生不良气味。腥臭卫矛在花开时苯乙腈急剧释放,浓度激增,可能苯乙腈达到一定浓度时,便由芳香性气味变为令人不愉悦的味道。
苯乙腈存在于腥臭卫矛的花苞分化到开花的整个过程中,V1和V2期含量极少,随着花瓣和花盘的分化,在V4期含量大量增加,但V4期的花并未呈现刺激性,说明此时苯乙腈仍未释放,直到V5期才经花盘中的外分泌结构释放出来。Konarska[26]发现,扶芳藤和欧洲卫矛(E. europaeus Linnaeus)的特化花托中都存在缺乏维管组织的光合蜜腺,花蜜通过蜜腺气孔(扶芳藤)或蜜腺气孔和分泌细胞壁(欧洲卫矛)流出。腥臭卫矛可能也存在类似的结构,苯乙腈随花蜜一起释放。
巨型虎杖(Fallopia sachalinensis Houtt.)、苹果(Malus pumila Mill.)等叶器官受到食草动物侵害时,会释放包括苯乙腈在内的挥发性混合物[27-28]。Yamaguchi等[29]证实,日本弧丽金龟(Popillia japonica Newman)侵染或茉莉酸甲酯(MeJA)处理巨型虎杖叶片时,由L-苯丙氨酸(L-phenylalanine)合成苯乙腈,细胞色素P450催化合成前体(E/Z)-苯乙醛肟(PAOx)向苯乙腈转化,CYP71AT96基因可能参与了苯乙腈的生物合成。与原变种石枣子相比,腥臭卫矛在开花期释放腥臭味的现象可能与调控苯乙腈合成的基因序列发生变异有关。
-
腥臭卫矛花序中不同发育时期的花及开花时花瓣和花盘挥发成分差异显著。PCA和HCA分析将腥臭卫矛花发育的5个时期与开花时(V5期)不同部位的挥发性成分被聚成4大类,V1和V2期属于叶醇型;V3与V4期聚为1类,成分复杂,化学型不明显;V5期和Y1为芳樟醇型;Y2属于苯乙腈型。腥臭卫矛开花期的刺激性气味主要来源于花绽开时(V5期)花盘释放的大量苯乙腈,与鱼腥草素无关。







 DownLoad:
DownLoad: