-
磷素是植物生长发育和产量提升所必需的大量营养元素之一,在植物生物合成、细胞分裂等生理生化过程中不可或缺[1]。土壤磷素主要是以有机磷和无机磷形式存在,磷素虽储量大,但绝大部分磷被土壤中铁铝离子等金属鳌合为难溶性磷酸盐不易被植物吸收利用[2-3]。为满足植物对磷的需求,往往需要施用大量磷肥,然而施用磷肥会带来环境污染且易被土壤固定,造成磷肥利用率低[4],当季利用率仅有10%~15%[5]。如何提高土壤磷库中磷素利用率,从而缓解土壤磷缺乏和大量施磷肥的矛盾是研究热点。解磷微生物在土壤磷循环过程中发挥着重要的作用,是破解土壤磷供应和植物磷需求矛盾的关注焦点。目前解磷微生物主要包括假单胞菌属(Pseudomonas)、芽孢杆菌属(Bacillus)、欧文氏菌属(Erwinia)、克雷伯氏菌属(Klebsiella)、沙雷氏菌属(Serratia)、曲霉菌属(Aspergillus)、青霉菌属(Penicillium)、小单胞菌属(Micromonospora)和链霉菌属(Streptomyces)等[6]。研究证实解磷微生物可通过释放有机酸、质子等酸解和酶解方式将土壤潜在磷库中的无效磷转化为有效磷供根系吸收而促进植物生长[7]。
毛竹(Phyllostachys edulis)主要分布于我国南方丘陵山区,是重要的经济竹种[6]。随着毛竹林面积扩大及间伐间隔期缩减,竹林资源质量下降,地力衰退,竹产业的可持续发展受到严重影响。研究显示,磷是毛竹林丰产和生态系统稳定性的首要限制因子之一[7]。因此,分离出高效解磷细菌是解决南方丘陵山区磷素供应不足的可行途径之一,研究团队从毛竹林土壤中筛选出1株具有解磷功能的产气肠杆菌(Enterobacter aerogenes),以往研究报道该菌株具有丰富的生物学特性,如具有ACC脱氨酶活性[6],但关于该菌株的解磷特性未深入研究,该菌株对毛竹生长及养分吸收的促进效果及机理尚不清楚,限制其进一步开发和利用。植物叶片的磷可分为无机磷、糖磷、核酸磷和残留磷4个组分,植株叶片的磷组分含量受环境的改变而发生改变,影响其叶片磷分配[8]。目前解磷微生物对植物叶片中不同形态磷组分的影响则未见报道。本研究拟在深入了解产气肠杆菌在不同碳、氮源及环境因子等条件下的解磷特性的基础上,通过盆栽试验评估该解磷细菌对毛竹实生苗的促生作用,明确该菌株对毛竹根系磷吸收和叶片磷组分积累的调控机制,以期为该菌株研制成生物肥料时发挥其最佳解磷功效,改善竹林土壤磷素营养提供依据,也有望揭示该解磷细菌对毛竹的促生机理,为毛竹专用生物肥料的应用提供参考。
-
产气肠杆菌由本课题前期从毛竹根际土壤中筛选的具有解磷功能菌株,基因登录号为KY780224,保存于江西农业大学森林保护实验室;毛竹实生苗栽培于学校温室大棚,苗龄90 d,温室平均温度28 ℃,湿度50%~70%,自然光照,竹苗试验期为当年4—10月份。基质土壤采自江西农业大学后山竹林红壤土,有机质含量6.37 g·kg−1,全磷含量0.24 g·kg−1,全氮含量0.43 g·kg−1。苗木培养介质为土壤:沙子:蛭石以2∶1∶1比例混匀。NB培养基:10 g蛋白胨,3 g牛肉膏和5 g氯化钠,蒸馏水1 000 mL;NBRIP培养基:5 g Ca3(PO4)2,10 g葡萄糖,0.1 g (NH4)2SO4,5 g MgCl2,0.25 g MgSO4·7H2O,0.2 g KCl,1 000 mL蒸馏水,pH 7.2~7.4。
-
选用Ca2(PO4)3作为唯一磷源,NBRIP培养基中其余成分不变,将蔗糖、葡萄糖、麦芽糖、果糖和可溶性淀粉以10 g·L−1的比例分别加入NBRIP液体培养基中作为单一碳源;NBRIP培养基其余成分不变,将(NH4)2SO4、KNO3、牛肉膏、蛋白胨、酵母粉以10 g·L−1的比例分别加入NBRIP液体培养基中作为单一氮源。在装有50 mL培养基的100 mL三角瓶中接种1%的接种量,加入产气肠杆菌种液。对照处理为不接菌,每个处理设置3个重复。在25 ℃条件下震荡培养3 d,发酵液离心10 min(4 ℃,10 000 r·min−1),采用钼锑抗比色法测定发酵液中有效磷含量[9]。
-
将10、20、30和40 mL的NBRIP培养基分别装入50 mL三角瓶,使装液量与三角瓶体积比分别为1/5、2/5、3/5和4/5;NBRIP培养基成分保持不变,利用稀盐酸将培养基初始pH分别调至1.5、2.5、3.5、4.5、5.5、6.5;分别按质量百分比浓度0%、1.0%、2.0%、3.0%、4.0%、5.0%、6.0%、7.0%、8.0%和9.0%向NBRIP培养基中加入氯化钠,保持培养基成分不变,均按1%接种量分别接入菌株种子液进行震荡培养。对照处理为不接菌,每个处理设置5个重复。在25 ℃条件下震荡培养3 d,发酵液离心10 min(4 ℃,10 000 r·min−1),采用钼锑抗比色法测定发酵液中有效磷含量[9]。
-
按1:100的接种比例,将菌株种子液分别接入以Ca3(PO4)2、FePO4、CaHPO4、AlPO4和植酸钙(Calcium phytate)为唯一不溶性磷源的NBRIP培养基中。对照处理为不接菌,每个处理设置5个重复,在25 ℃条件下震荡培养3 d,发酵液离心10 min(4 ℃,10 000 r·min−1),提取上清液后采用钼锑抗比色法测定发酵液中可溶性磷含量[9]。
-
在产气肠杆菌活化后,用接种环挑取少量菌体于NBRIP培养基中,28 ℃、180 r·min−1振荡培养48 h。发酵液离心5 min(4 ℃,5 000 × g),将菌体在0.85%无菌生理盐水中润洗3次,并调整菌悬液(108 cfu·mL−1),制成液体菌剂;采用灌根法分别施用在毛竹根际土壤中,以等量无菌生理盐水作为对照处理,每株20 mL施用量(每个花盆种植1株),花盆内径18 cm,盆高度18 cm,每盆添加培养介质约3.0 kg。采用随机区组设计试验,每组处理均为4盆,设置5个重复,各处理均20盆(株)。期间按时浇水,不添加任何肥料,正常管理。在温室培养180 d后,每个处理组内随机取1盆(株),累计每处理取5盆(株),选择晴朗的上午采用LI-6400光合仪测定毛竹叶片净光合速率;将毛竹从塑料花盆中取出并洗净,用于测定毛竹苗高、地径,在105 ℃条件下杀青30 min,75 ℃ 烘干至恒质量,测定其叶片生物量和总生物量,采用抖落法采集根际土壤[10],用于测定土壤有效磷、铵态氮和硝态氮含量;收集所有根系,洗净备用。用球磨仪粉碎毛竹根系和叶片,过孔径0.5 mm筛,用于测定根系和叶片全磷含量以及叶片不同磷功能组分含量。根据Close等[11]的提取方法,用三氯乙酸(TCA)提取毛竹叶片不同形态的磷功能组分,测定无机磷、糖磷、核酸磷和残留磷4种磷组分含量。土壤和植物组织其他养分测定方法见参考文献[12]。
-
采用Origin 8.5和SPSS 13.0软件进行数据处理和统计分析,采用单因素多重比较(Duncan法)分析解磷特性试验数据,采用配对T检验分析促生长作用试验数据(P<0.05),采用Pearson法进行相关性分析。图表中数据均为平均值±标准差(n=5)。
-
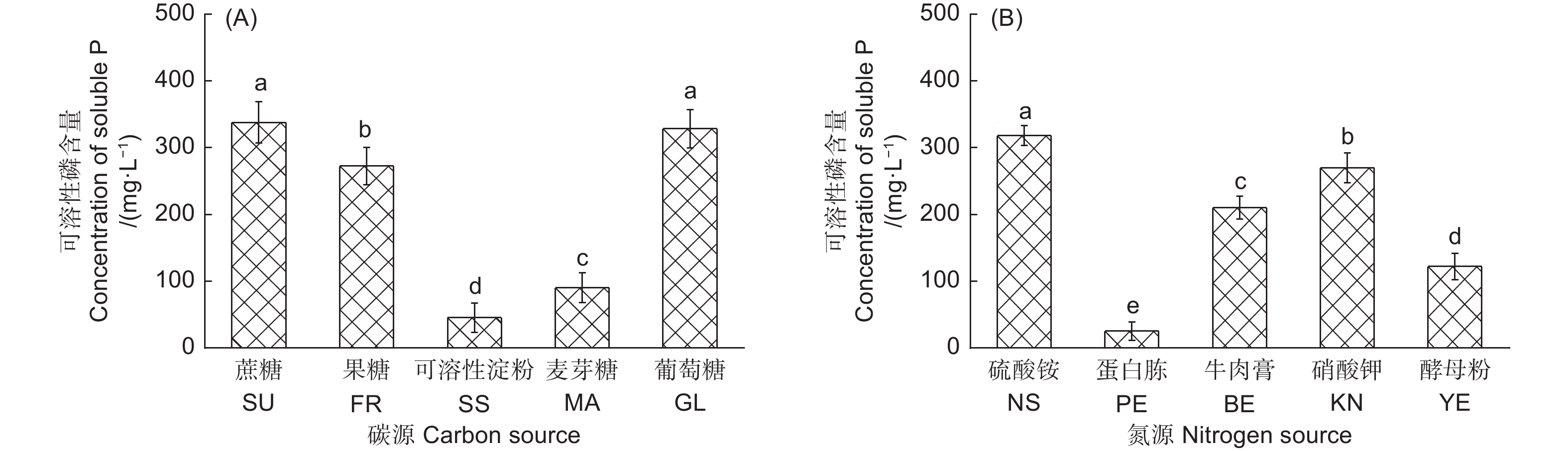
不同碳源对产气肠杆菌溶解Ca2(PO4)3能力的影响显著(图1A)。以葡萄糖和蔗糖为唯一碳源时,该菌株的溶解能力最强,分别为328.41 mg·L−1和337.76 mg· L−1;其次为果糖,解磷量为272.59 mg·L−1;以可溶性淀粉为碳源时,产气肠杆菌的解磷能力最低,为45.32 mg·L−1。不同氮源对产气肠杆菌的解磷能力也影响显著(图1B)。以硫酸铵为唯一氮源时,该菌株的解磷能力最强,为318.42 mg·L−1;其次为硝酸钾,解磷量为270.3 mg·L−1;以蛋白胨为氮源时,该菌株解磷能力最低,解磷量仅为25.25 mg·L−1。
-
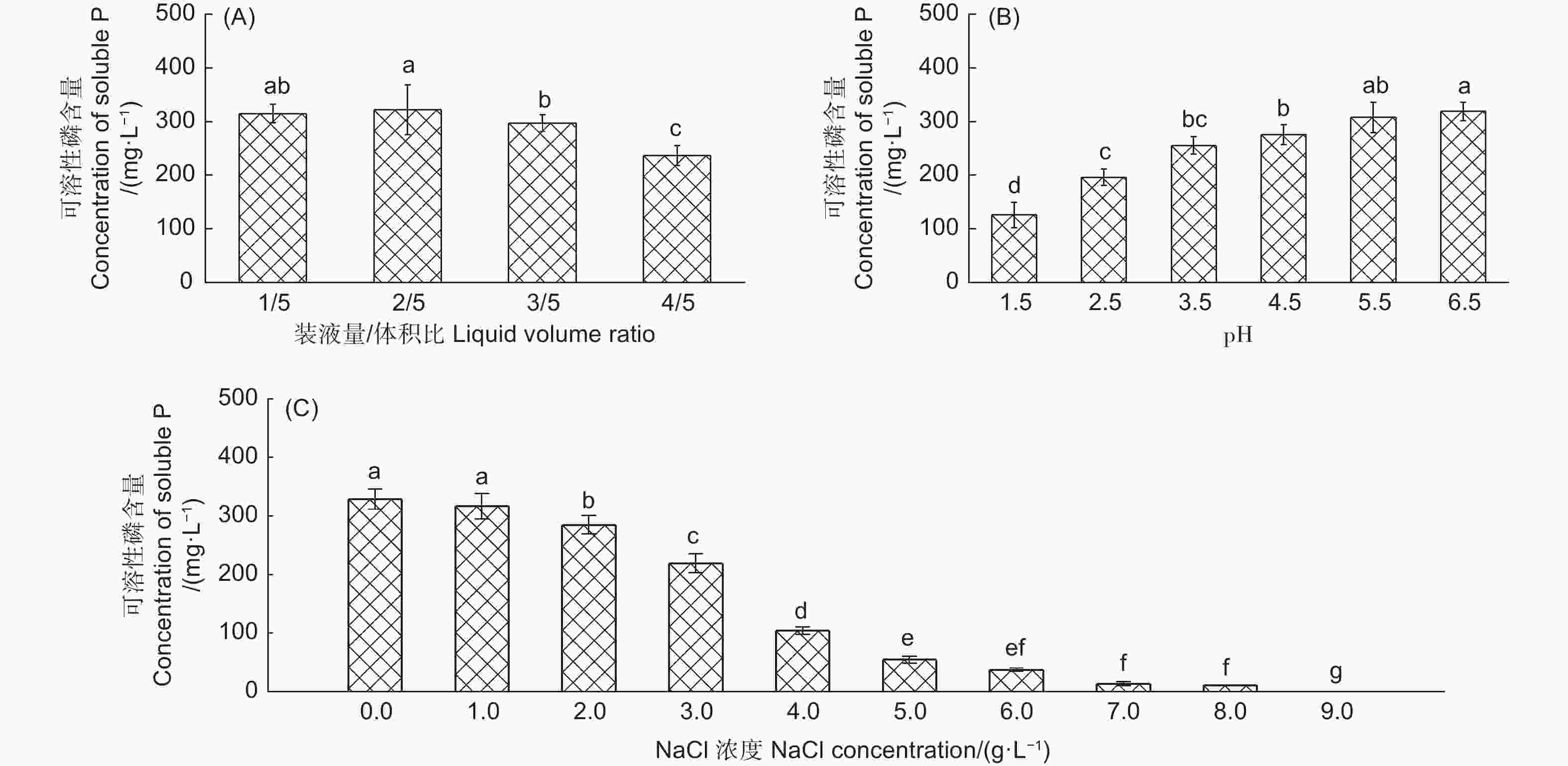
不同环境条件下产气肠杆菌对Ca2(PO4)3的溶解能力有显著差异(图2)。在装液量/体积比为1/5和2/5时该菌株具有较强的解磷能力,解磷量分别为314.92 mg· L−1和321.41 mg·L−1,装液量/体积比为3/5和4/5时,该菌株也具有较好的解磷能力,解磷量分别为297.16 mg·L−1和236.43 mg·L−1(图2A)。初始pH对产气肠杆菌的解磷能力有显著影响,初始pH值越大,解磷能力越强,当初始pH值5.5和6.5时,该菌株具有较强的解磷能力,解磷量分别为307.2 mg·L−1和318.82 mg·L−1,其次为初始pH值4.5、3.5和2.5时,在初始pH值1.5时解磷能力最低,解磷量为125.6 mg·L−1(图2B)。随着NaCl浓度升高,产气肠杆菌的解磷能力呈下降趋势,在NaCl浓度为0和1.0 g·L−1,该菌株解磷能力最大,解磷量分别为331.45 mg·L−1和328.72 mg·L−1,显著高于其它处理(图2C)。
-
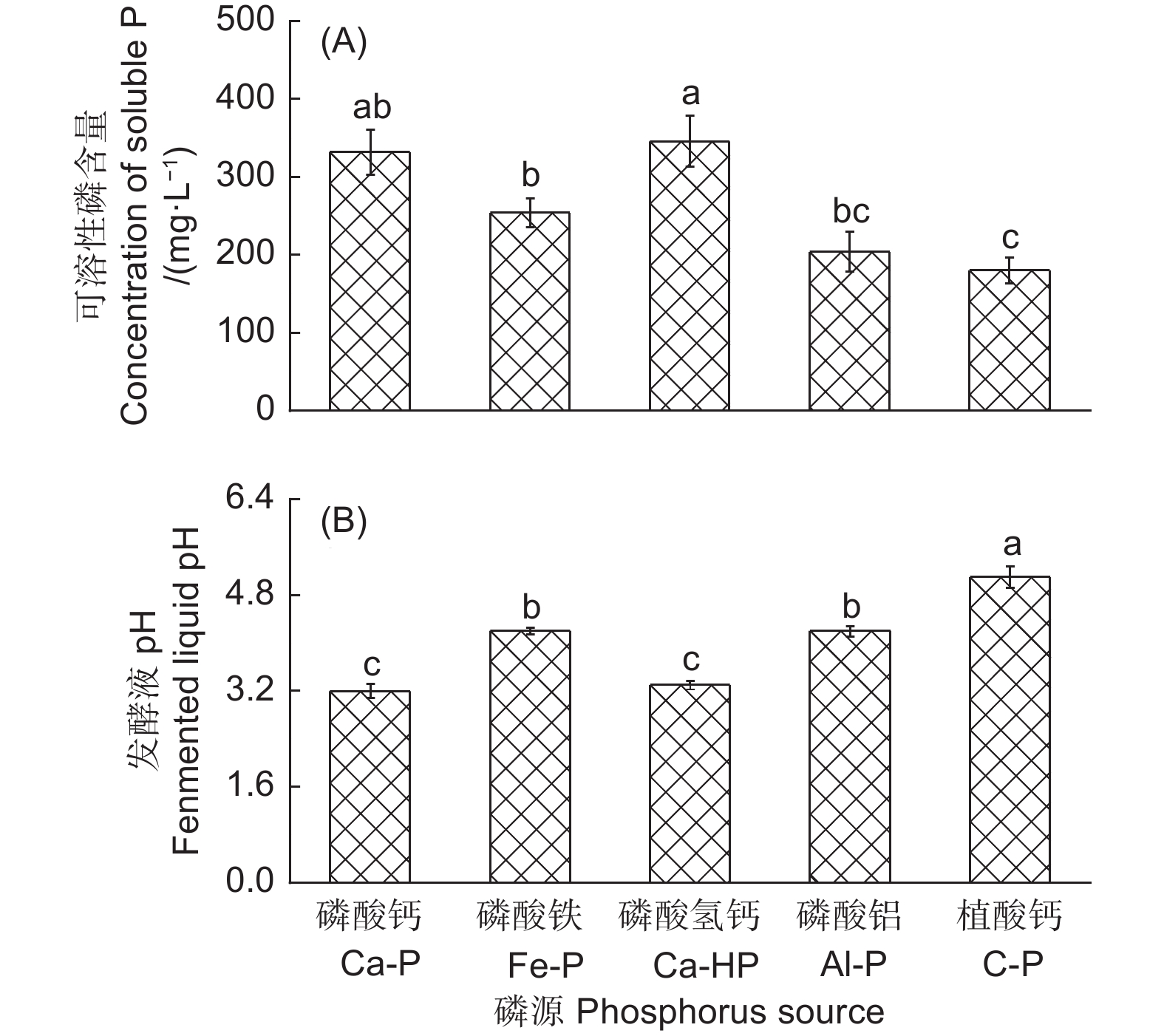
产气肠杆菌对5种难溶性矿质盐的溶解能力有显著差异(图3)。该菌株对CaHPO4和Ca2(PO4)3的溶解能力最强,解磷量分别为345.91 mg·L−1和331.83 mg·L−1,对FePO4和AlPO4也具有较好的溶解能力,其解磷量分别为253.61 mg·L−1和203.65 mg·L−1,对植酸钙的溶解能力最弱,其解磷量为179.63 mg·L−1(图3A)。在对5种磷源的溶解过程中,培养基pH均显著下降(图3B)。相关性分析表明,发酵液pH和产气肠杆菌对不同磷源的解磷量呈显著负相关(r=−0.54, n=25)。
-
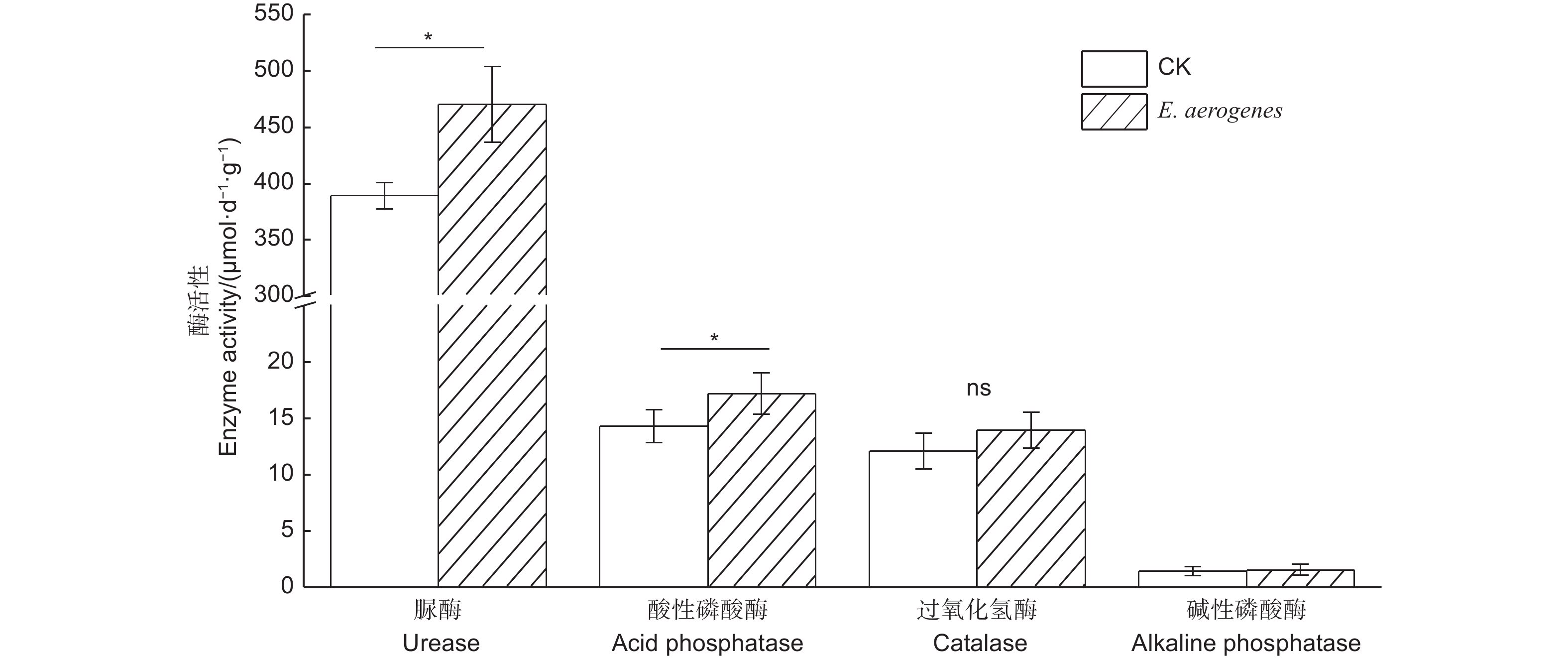
与对照相比,施用产气肠杆菌显著提高了土壤中有效磷含量,增幅为50.6%,而对根际土壤铵态氮和硝态氮含量无显著影响(表1)。施用菌剂显著增强了土壤酸性磷酸酶和脲酶活性,增幅分别为20.6%和21.0%;而对碱性磷酸酶和过氧化氢酶无显著影响(图4)。
观测指标
Observed variable对照
Control施用菌剂
Adding strain有效磷 Available P/(mg·kg−1) 4.62±0.38 b 6.96±0.45 a 铵态氮 Ammonium N/(mg·kg−1) 0.97±0.13 a 1.14±0.16 a 硝态氮 Nitrate N/(mg·kg−1) 2.11±0.25 a 2.42±0.28 a 注:同行不同字母表示差异显著(P<0.05),下同。
Notes: Different letters in the same row mean significant difference at 0.05 level. The same below.Table 1. Effect of adding Enterobacter aerogenes on soil available nutrients of moso bamboo seedlings
-
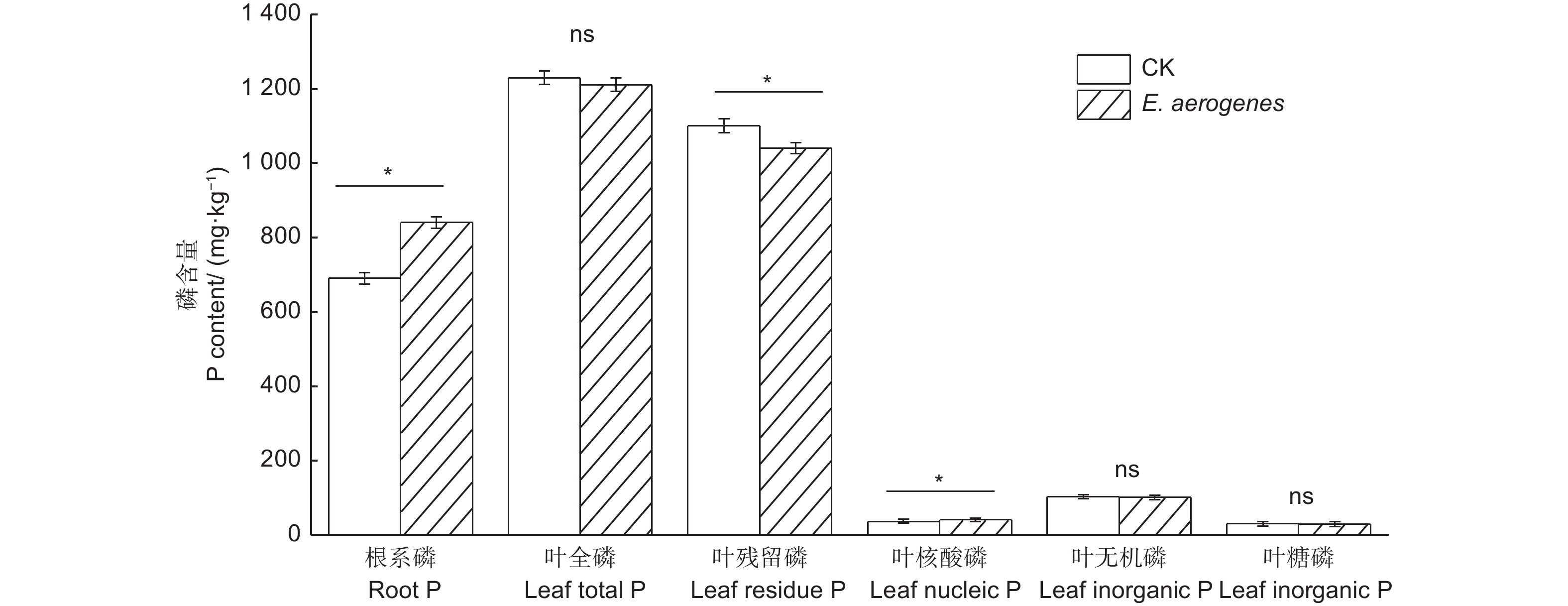
与对照相比,施用产气肠杆菌显著提高了根系磷和叶片核酸磷含量,增幅分别为21.7%和11.6%,显著降低了叶片残留磷含量,降幅为5.5%;而对叶片全磷、无机磷和糖磷含量无显著影响(图5)。与对照相比,施用产气肠杆菌菌剂显著提高毛竹实生苗的苗高、地径、生物量和叶片净光合速率,增幅分别为30.9%、23.4%、44.4%和42.1%(表2)。
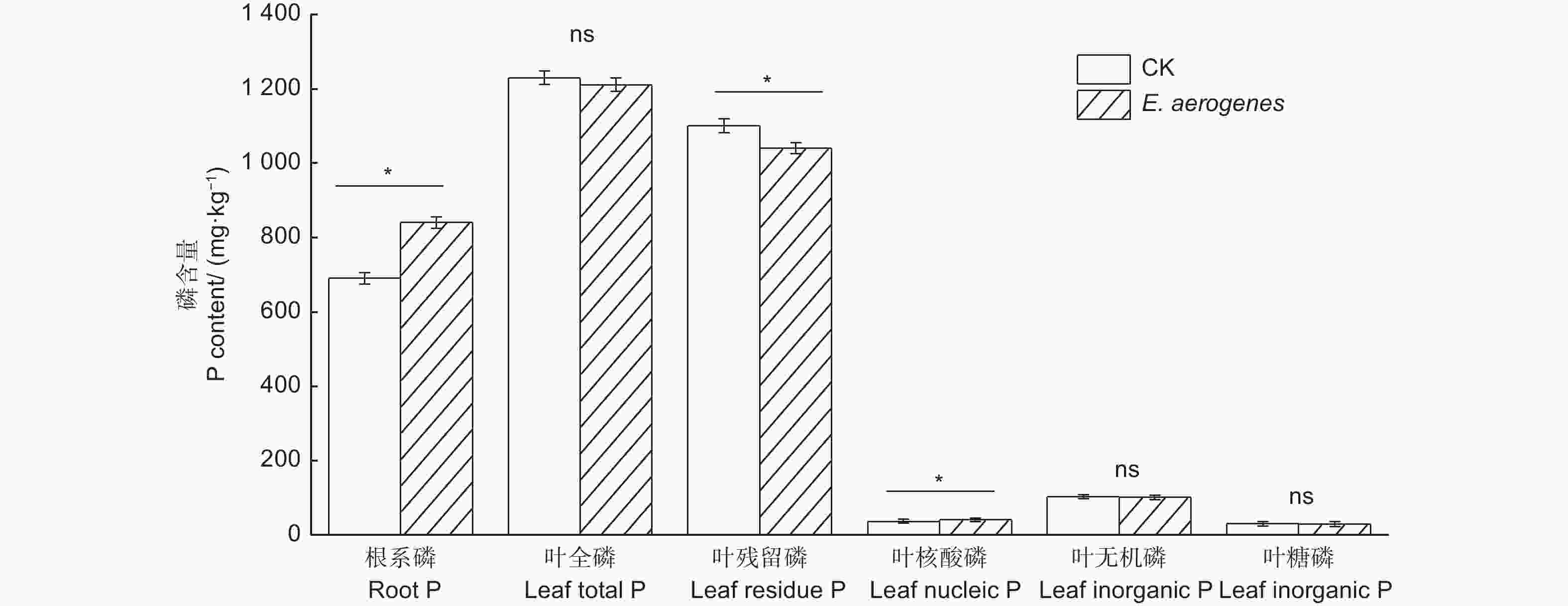
Figure 5. Effect of Enterobacter aerogenes inoculation on root total phosphorus and leaf phosphorus functional fractions of moso bamboo seedlings
观测指标
Observed variable对照
Control施用菌剂
Adding strain苗高 Seeding height/cm 22.42±0.65 b 29.36±1.27 a 地径 Ground diameter/cm 1.49±0.08 b 1.84±0.09 a 总生物量 Total biomass/g 3.62±0.21 b 5.23±0.13 a 净光合速率
Net photosynthetic rate/(μmol·m−2·s−1)2.57±0.36 b 3.65±1.86 a Table 2. Effect of adding Enterobacter aerogenes on the growth variables of moso bamboo seedlings
-
Pearson相关性分析结果显示,叶片磷组分与土壤理化性质和生长指标相关性不尽一致,其中叶无机磷和叶糖磷与土壤理化性质和生长指标并无相关性,叶核酸磷与土壤有效磷、土壤酸性磷酸酶、植株净光合速率、植株生物量、叶生物量和根系全磷呈显著正相关;叶残留磷与土壤有效磷、土壤脲酶、植株净光合速率、植株生物量、叶生物量和根系全磷呈显著负相关;叶全磷、pH与核酸磷呈显著负相关,与残留磷呈显著正相关(表3)。
观测指标
Observed variable叶无机磷
Leaf inorganic P叶糖磷
Leaf sugar P叶核酸磷
Leaf nucleic P叶残留磷
Leaf residue P铵态氮 Ammonium N/ (mg·kg−1) 0.466 0.041 0.631 −0.607 硝态氮 Nitrate N/(mg·kg−1) 0.160 −0.461 0.628 −0.569 有效磷 Available P/(mg·kg−1) −0.130 −0.613 0.948** −0.961** pH值 pH value 0.253 0.217 −0.829* 0.895* 矿质氮 Mineral N/(g·kg−1) 0.282 −0.342 0.701 −0.647 叶全磷 Leaf total P/(g·kg−1) 0.234 0.432 −0.951** 0.979** 根系全磷 Root total P/(g·kg−1) 0.113 −0.321 0.927** −0.924** 叶生物量 Leaf biomass/g −0.291 −0.279 0.925** −0.941** 植株生物量 Plant biomass/g −0.252 −0.445 0.881* −0.937** 脲酶 Urease/(nmol·g−1) −0.666 −0.242 0.758 −0.848* 酸性磷酸酶 Acid phosphatase/(nmol·g−1) 0.314 −0.626 0.853* −0.788 碱性磷酸酶 Alkaline phosphatase/(nmol·g−1) −0.680 0.500 −0.055 −0.081 过氧化氢酶 Catalase/(nmol·g−1) −0.028 −0.841* 0.803 −0.777 净光合速率 Net photosynthetic rate/(μmol·m-2·s−1) 0.037 −0.359 0.949** −0.948** 注:*代表差异显著(P<0.05); **代表差异极显著(P<0.01)。
Notes: * means significant difference at P<0.05 level; ** means significant difference at P<0.01 level.Table 3. Correlation coefficients between leaf phosphorus fractions and each measured index
-
碳、氮源是解磷微生物必不可少的能源物质,不同种类的碳、氮源对解磷微生物生长代谢及溶解难溶性磷酸盐的能力有着显著影响[13]。研究显示,解磷微生物可通过葡萄糖氧化代谢途径分泌有机酸来溶解难溶性磷酸盐[14],分泌物中的某些糖成分可作为信号分子提高细菌磷酸酶相关基因的表达[6]。本研究结果发现,产气肠杆菌在碳源为蔗糖或葡萄糖时对磷酸钙的溶解效果最好,推测可能是该菌株能更有效的吸收利用这2种糖分来提高磷酸酶相关基因的表达,或进入氧化代谢途径,进而更好地促进溶解难溶性磷源。氮是构成微生物细胞核酸和蛋白质等遗传物质的重要元素,对解磷微生物的生长繁殖和代谢也有着重要的影响[7]。Wenzel[15]发现解磷细菌在氮源为NH4+时对Ca2(PO4)3具有较强的溶解能力,而氮源为NO3−对Ca2(PO4)3几乎没有溶解能力。本研究结果显示,产气肠杆菌在氮源为NH4+和NO3−时对磷酸盐均具有较好的溶解能力,这表明该菌株对氮源的可利用范围更宽泛,适应性也较强。以往研究表明,培养基中盐浓度、初始pH值和接种量均能显著影响解磷微生物对难溶性磷源的溶解能力[16-17]。Zeng[18]对解磷细菌JW-SD2进行解磷特性研究发现,不同装液量、初始pH值和不同盐浓度对该菌株的解磷能力有着显著影响,证实了环境因子对解磷微生物有显著的影响。我国南方丘陵山区土壤环境因子较为复杂,如土壤酸度和氧气浓度变化较大,本研究结果显示,产气肠杆菌具有良好的耐酸性和耐盐性等功效,具有适应不同土壤环境因素的潜力。
解磷微生物的解磷机制不尽相同,有学者认为解磷微生物可通过分泌有机酸(草酸、苹果酸、柠檬酸、葡萄糖酸等)降低土壤pH,促进难溶性磷的溶解[19],也有学者认为解磷微生物能与土壤中Fe3 + 、Al3 + 、Ca2 + 等阳离子螯合,通过与磷酸根离子竞争土壤中相同的吸附位点,释放磷酸根离子[16]。此外,还有部分学者认为解磷微生物可以通过释放磷酸酶活化土壤有机磷,使固定态磷向生物有效态磷转变[20]。本试验结果发现产气肠杆菌对无机磷源钙磷、铁磷、铝磷和有机磷源植酸钙等磷源的溶解有显著差异,在溶解这5种不同磷源过程中,发酵液pH也存在显著差异,这和以往部分研究结果一致[21],推测该菌株对不同类型的难溶性磷酸盐可能具有不同的溶解机制。解磷微生物可通过分泌有机酸和质子络合来溶解难溶性钙盐,如Wei等[22]发现部分解磷细菌主要通过分泌草酸来溶解土壤中难溶性磷源。此外,解磷微生物也能通过络合作用来溶解铁磷和铝磷[1]。本试验产气肠杆菌对这5种不同难溶性磷酸盐的溶解能力差异是否因分泌有机酸或各种酶类的差异还有待进一步研究。毛竹主要分布于我国南方丘陵地区,该地区土壤中无机态磷主要以闭蓄态铁磷和铝磷为主,钙磷次之,本试验中产气肠杆菌对难溶性无机磷和有机磷均有较好的溶解能力,显示该菌株对不同类型土壤具有一定的适用性,这为今后该菌株应用于不同土壤类型发挥其解磷功效提供了潜能,也具备成为今后开发竹林生物肥料提高优良解磷菌株的潜力。
-
土壤磷有效性能显著影响植物根系发育和植物生长[23],解磷菌能够提高土壤磷有效性和植物吸磷量,提高植物生物量,在土壤磷循环过程中有着重要调控作用[24]。本试验结果显示,施用产气肠杆菌能显著提高土壤有效磷养分,增加毛竹根系磷含量,提高毛竹实生苗生物量,这为今后开发竹林生物肥料提供优良的解磷菌株。诸多研究指出,解磷微生物对植物有较好的促生效果,然至今促生机理尚不明确[25]。部分报道解磷微生物可直接分泌小分子有机酸,通过酸类物质的羧基与金属离子发生螯合作用和产生磷酸酶类物质提高土壤磷素利用率,促进植物生长[26],也有部分研究结果表明,解磷微生物通过调控植物根际土壤有效养分来影响植株地上部分养分含量,而不是依靠溶磷微生物溶解土壤中难溶性磷源[27]。本实验结果显示施用解磷细菌产气肠杆菌能显著增强土壤中磷酸酶和脲酶活性,说明解磷菌剂能通过影响土壤酶活来提高土壤有效磷含量,这也验证以往研究中证实的解磷菌可通过改变土壤酶活来提高土壤磷有效性[28],而该菌株能否通过酶活性来影响土壤铵态氮和硝态氮含量还有待于进一步长期观测。毛竹主要分布我国南方丘陵山区,该地区多为酸性红壤,土壤有效磷含量较低,使得磷素成为限制毛竹林生产力提升的主要因子[29]。施用产气肠杆菌显著提高毛竹根际土壤有效磷、土壤酸性磷酸酶、根系磷含量,说明施用解磷菌株产气肠杆菌能通过影响土壤有效养分和竹苗养分促进其生长发育。此外,施用产气肠杆菌能显著提高毛竹实生苗根系磷含量,对毛竹叶片全磷含量无显著影响,说明施用解磷菌剂能提高毛竹对磷的吸收利用,可是仍难以满足毛竹高速生长过程中对磷的需求,推测毛竹生物量的增加过程中稀释了叶片磷素的含量,从而导致毛竹叶片中磷素含量无显著变化。
为适应低磷土壤,植物会对叶磷组分再分配来维持其较高的磷利用效率和光合作用[7, 30]。以往研究结果显示,解磷菌能提高植物叶片磷含量[27],而对叶片中不同磷组分含量有何影响却尚未见报道。无机磷和糖磷是植物叶片代谢活跃的磷,核酸磷是植物组织中最大的有机磷库,参与遗传信息和蛋白质的合成,是植物进行再分配的主要磷库[31]。在缺磷土壤的森林生态系统中,植物会调节叶片中不同磷组分重新分配,以维持光合作用效率,适应不同低磷土壤环境[30]。Schachtman[32]发现,植物叶片中核酸态磷被用于维持细胞内的胞质磷含量,随着植物生长速度变化,胞质磷能显著地影响叶片的光合作用速率。本研究发现施用解磷菌剂毛竹叶片核酸态磷含量显著提高,残留磷含量显著降低,核酸态磷与毛竹叶片净光合速率呈显著正相关性,这表明在土壤磷限制下,产气肠杆菌能提高叶片中用于蛋白质和酶合成的核酸磷含量,促进残留磷向核酸磷转化,保证叶片光合作用及其生理需求,在低磷的土壤环境中维持相对稳定的光合速率。目前需要进一步的试验来阐明叶片残留磷组分的功能作用,用以解释在本研究中施用解磷菌剂导致净光合速率与残留磷呈负相关性。相关分析表明,土壤有效磷、毛竹根系全磷、生物量与叶片中核酸磷含量呈显著正相关,与残留磷呈显著负相关,表明施用产气肠杆菌能有效调控毛竹叶片中不同磷组分含量来促进植株磷利用效率,进而促进毛竹的生长和植株生物量的提高。以往研究也证实植物可通过调控叶片磷组分含量来适应缺磷土壤[7]。Mo[33]等人通过野外控制试验证实施用磷肥能提高植物叶片的无机磷、糖磷和核酸磷含量,植物调控叶片中不同磷组分含量的分配来满足光合作用对磷的需求。本试验结果揭示了土壤解磷细菌产气肠杆菌对毛竹根际土壤有效养分含量和叶片磷组分特征的影响,在解磷细菌对毛竹的促生机制方面提供了解释,为改善毛竹林土壤有效养分利用提供了参考,也为今后生物磷肥的开发及竹林可持续发展提供理论依据。
Phosphate Solubilizing Characteristics of Enterobacter aerogenes and Its Growth-promoting Effect on Phyllostachys edulis Seedlings
- Received Date: 2021-10-09
- Accepted Date: 2021-12-09
- Available Online: 2022-06-20
Abstract:








 DownLoad:
DownLoad: